被骗了, Cut Bank, MT, USA
1天前 •reported by user-hfvt2146 • 细节
来自埃斯特·坎特雷尔的未订购项链, Virginia Beach,
1天前 •reported by user-xwkhn648 • 细节
Facebook诈骗, Oakland, California, USA
3天前 •reported by user-hbbz8669 • 细节
我不是从一家公司订购的空包裹, Chicago, IL, USA
5天前 •reported by user-kctpp117 • 细节
当我打开它时-它是空的。
我用谷歌搜索了这家公司,发现了很多 “骗局” 评论。我不记得从这家公司订购过的商品。
风险声明:使用 Alkaloids Chewable Tablets—White Vein 产品可能导致消费者摄入超过预期的剂量,这可能导致不良健康影响。
Shaman Botanicals, LLC 尚未收到与此批次 Alkaloids Chewable Tablets—White Vein 相关的不良事件报告。
受影响产品:
- 包装在 2 片装袋中
- UPC 代码 810057763724
- 20 片装袋,UPC 代码…
Dream Bone, Tampa, FL, USA
1周前 •reported by user-qbzb4927 • 细节
Shinerya.com 诈骗, Tahlequah, OK, USA
3周前 •reported by user-mjjyc942 • 细节
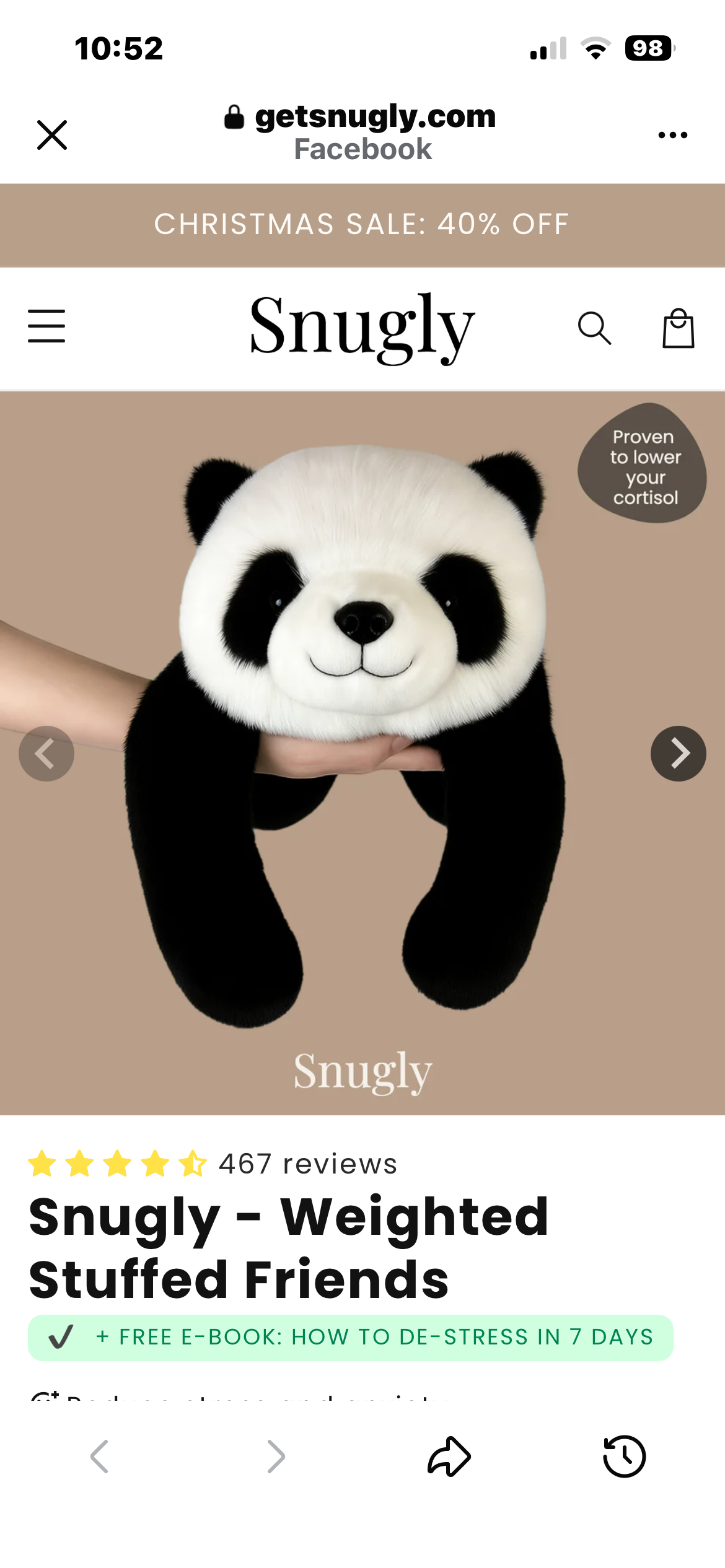
欺诈 Koala Snugly, Las Vegas, NV, USA
2个月前 •reported by user-vdxkg271 • 细节
据说是美国原来是中国
假冒的 USPS 假冒标签名称:Honeas Orxty, 290 Duffy Ave, Hicksville, NY 11801, USA
2个月前 •reported by user-vbnw3125 • 细节
电子邮件通知完全来自 addly