Recall notice
EzriCare & Delsam Pharma Artificial Tears - recalled due to Potential microbial contamination, USA
2 years ago •source fda.gov
United States
Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops, distributed by /EzriCare, LLC- and Delsam Pharma, to the consumer level, due to possible contamination. The Centers for Disease Control and Prevention (CDC) alerted FDA to an investigation of a multi-state cluster of Verona Integron-mediated Metallo-β-lactamase (VIM)- and Guiana-Extended Spectrum-β-Lactamase (GES)- producing carbapenem-resistant Pseudomonas aeruginosa (VIM-GES-CRPA) infections possibly associated with the use of the artificial tears manufactured by Global Pharma Healthcare. To date, there are 55 reports of adverse events including eye infections, permanent loss of vision, and a death with a bloodstream infection. The product was distributed Nationwide in the USA over the Internet.Risk Statement: Use of contaminated artificial tears can result in the risk of eye infections that could result in blindness.
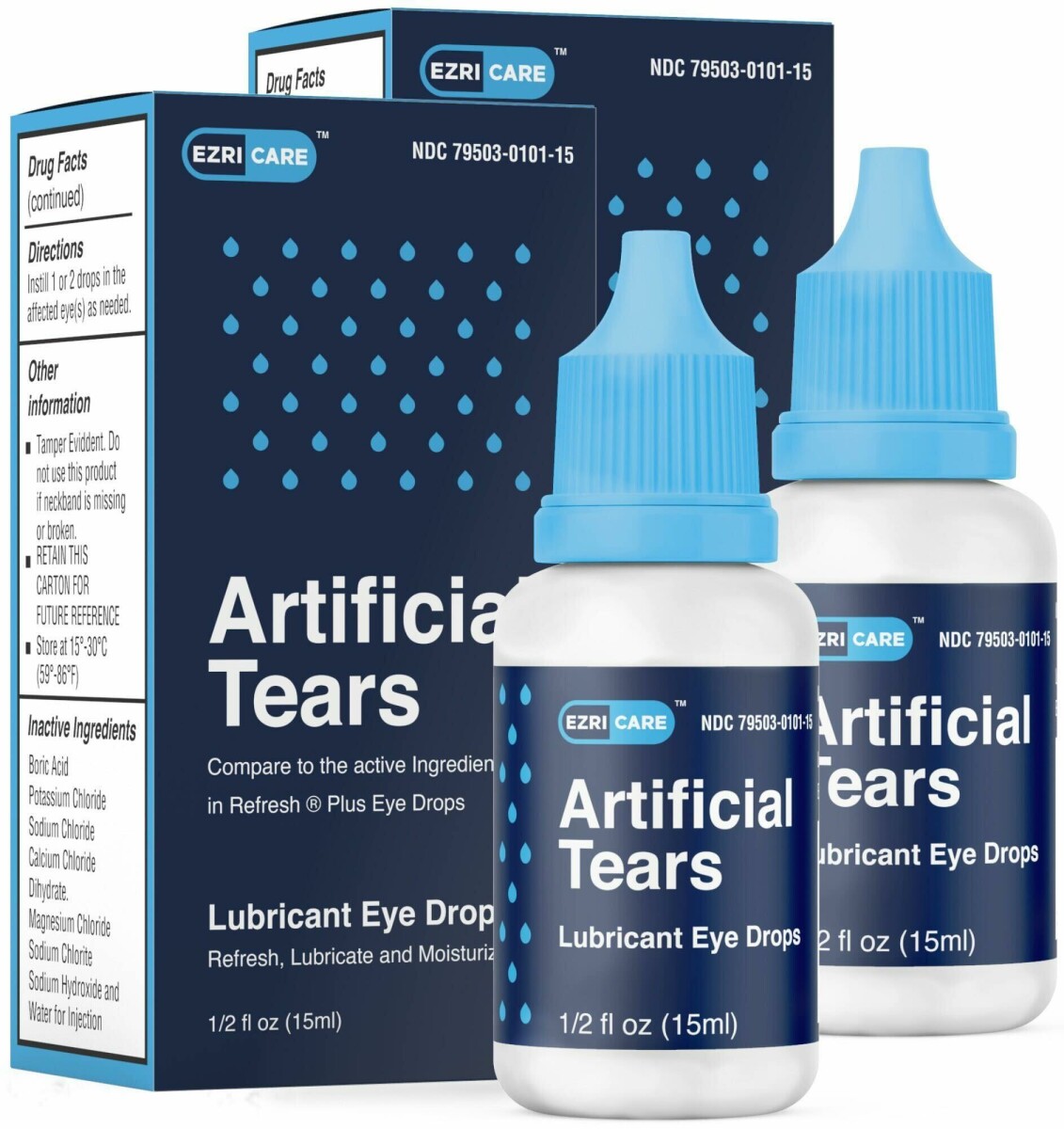
Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml) bottle are used as a protectant against further irritation or to relieve dryness of the eye for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun. The product is packaged in a bottle with a safety seal and are placed in a carton box. It can be identified by the photos below.
- Ezricare NDC 79503-0101-15. UPC 3 79503 10115 7
- Delsam Pharma's NDC 72570-121-15. UPC -72570-0121-15.
Global Pharma Healthcare is notifying the distributors of this product, Aru Pharma Inc. and Delsam Pharma and is requesting that wholesalers, retailers and customers who have the recalled product should stop use.
Consumers with questions regarding this recall can contact the distributors: Aru Pharma/Ezricare, LLC. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these over-the-counter drug products.
Company name: Global Pharma Healthcare
Brand name: EzriCare & Delsam Pharma
Product recalled: Artificial Tears Lubricant Eye Drops
Reason of the recall: Potential microbial contamination
FDA Recall date: February 02, 2023
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/global-pharma-healthcare-issues-voluntary-nationwide-recall-artificial-tears-lubricant-eye-drops-due
312
Comments
Comment