Recall notice
Ropivacaine Hydrochloride Injection recalled due to Inert Fiber, USA
8 months ago •source fda.gov
United States
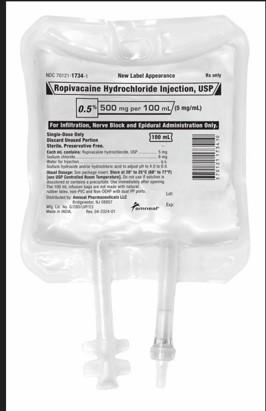
Amneal Pharmaceutical LLC is recalling two lots of Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, Infusion bags to the hospital/user level as the products may contain an inert fiber identified as polypropylene fibers from the IV bag. The recalled product was distributed nationwide to wholesalers/distributors between the dates of 04/23/2024 to 11/8/2024 only.Risk Statement: Introduction of polypropylene particulates into the epidural space (or inadvertent administration into the intrathecal space) may result in a variety of adverse events. There is a reasonable probability that particulate matter in the epidural space may cause an epidural inflammatory process to meningitis or potentially damage the spinal cord. Administered intrathecally, particulate matter could result in inflammation, hydrocephalus (water on the brain), which could lead to embolization and organ damage.
The product is indicated for the production of local or regional anesthesia for surgery and or acute pain management and is packaged in 12x100mL Single Dose IV bags (NDC 70121-17343). The affected Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, products are Lot AL240003 (exp 01/2026) and Lot AL240004 (exp 01/2026). No other Ropivacaine Hydrochloride Injection, USP lots are impacted.
Amneal is notifying its customers by UPS and is arranging for the return of all recalled products. Wholesalers/distributors are asked to notify their hospital/ user customers of the recall and provide instructions to contact Amneal for the return of the recalled products to Amneal.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/amneal-pharmaceutical-llc-issues-nationwide-recall-ropivacaine-hydrochloride-injection-usp
Comments
Comment