Recall notice
Olympus Single use ligating device recalled due to PolyLoop Device fail to detach, USA
2 months ago •source fda.gov
United States
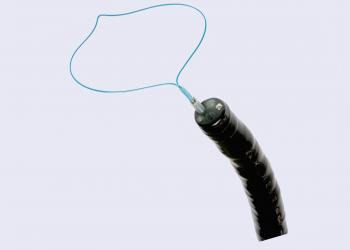
Olympus Corporation today announced a voluntary medical device corrective action for its Single-Use Ligating Device ("Polyloop") following identification of a potential safety issue. The affected product is distributed nationwide in the United States.The Polyloop device is designed for use with Olympus endoscopes to deliver a nylon loop snare intended to prevent or control bleeding following polypectomy of pedunculated polyps.
Olympus took this action after receiving adverse event reports indicating that the Polyloop may fail to release or detach as expected during use, resulting in the loop becoming unintentionally anchored around patient anatomy.
An unreleased ligation loop can present procedural challenges, requiring emergency intervention for removal from patient's anatomy. Risks include bleeding, mucosal injury, perforation, and in severe cases, the need for surgical intervention or hospitalization.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/olympus-announces-voluntary-corrective-action-single-use-ligating-device
Comments
Comment