Recall notice
Nova-Tech Lactate Ringers Injection recalled due to Presence of fiber-like particles, USA
11 months ago •source fda.gov
United States
Nova-Tech, Inc. is voluntarily recalling one (1) lot of Lactated Ringers Injection for Animal Use Only to the end user level. The Lactated Ringers Injection has been found to potentially contain fiber-like visible particles. Lactated Ringers Injection was distributed nationwide to wholesale distributors. The issue was discovered by Nova-Tech during visual inspection for stability testing.Risk Statement: Intravenous administration of an injectable product that contains particulate matter may result in serious adverse events. Potential complications related to the injection of particles include inflammation of a vein, granuloma, and blockage of blood vessels in the heart, lungs, or brain which can cause stroke or life-threatening blood clot events, including death. The frequency and severity of these adverse events could vary depending upon a variety of factors including the size and number of particles in the drug product, patient comorbidities (such as age, and compromised organ function), and presence or absence of vascular anomalies.
Some possible signs and symptoms of an adverse event include pain, weakness, swelling, paralysis, fever, labored or fast breathing, vomiting, decreased activity level, vocalization, or loss of consciousness. If you observe any of these or any other concerning signs in an animal that may have been administered this product, please contact a veterinarian as soon as possible.
AFFECTED PRODUCT:
- The product was distributed between December 31st, 2024 and February 28th, 2025.
- The product is used as an injectable solution as directed by a veterinarian
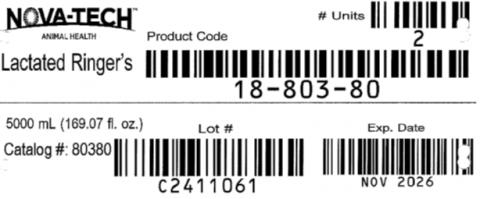
- Is packaged in 5-liter IV bags with 2 units per case
- Unit UPC 365207803803.
- The affected Lactated Ringers Injection 5- Liter is lot # C2411061
- Expiration date NOV 2026.
- The product can be identified by item code 18- 803-80, NDC # 65207-803-80.
Consumers should contact their veterinarian if they have experienced any problems that may be related to taking or using this drug product.
Comments
Comment