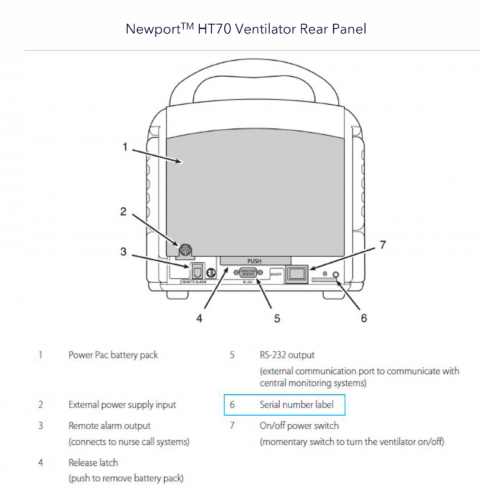
Recall notice
Newport™ HT70 variant ventilators and service parts recalled due to Potential Defect, USA
8 months ago •source fda.gov
United States
Medtronic issued a voluntary recall notification to global customers related to specific Newport™ HT70 and HT70 Plus ventilators and certain related Newport™ service parts. The FDA recently designated this voluntary action by Medtronic as a Class I recall. With this recall, Medtronic is advising the discontinuation of clinical use of the affected devices. Investigation into customer complaints identified two separate capacitors on one of the ventilator's controller Printed Circuit Board Assembly (PCBA), that, in case of failure, may result in:- The ventilator shuts down during use, or
- The shutdown alert alarm is failing to sound effectively.
The following table identifies the item name, manufacture date, and use by date:
No instances of both capacitors failing on the same PCBA board have occurred, nor are they anticipated to occur.
If a ventilator fails and does not provide adequate ventilation, the patient may not be able to breathe on their own, leading to low oxygen levels, high carbon dioxide levels, and potentially severe consequences like brain injury or death. There have been 63 medical device reports (MDRs) associated with this issue, including two serious injuries and one death. HT70 and HT70 Plus ventilators are intended for use by home users, as well as for infant and pediatric patients who may be at higher risk of injury or death due to unanticipated ventilator failures.
Comments
Comment