Recall notice
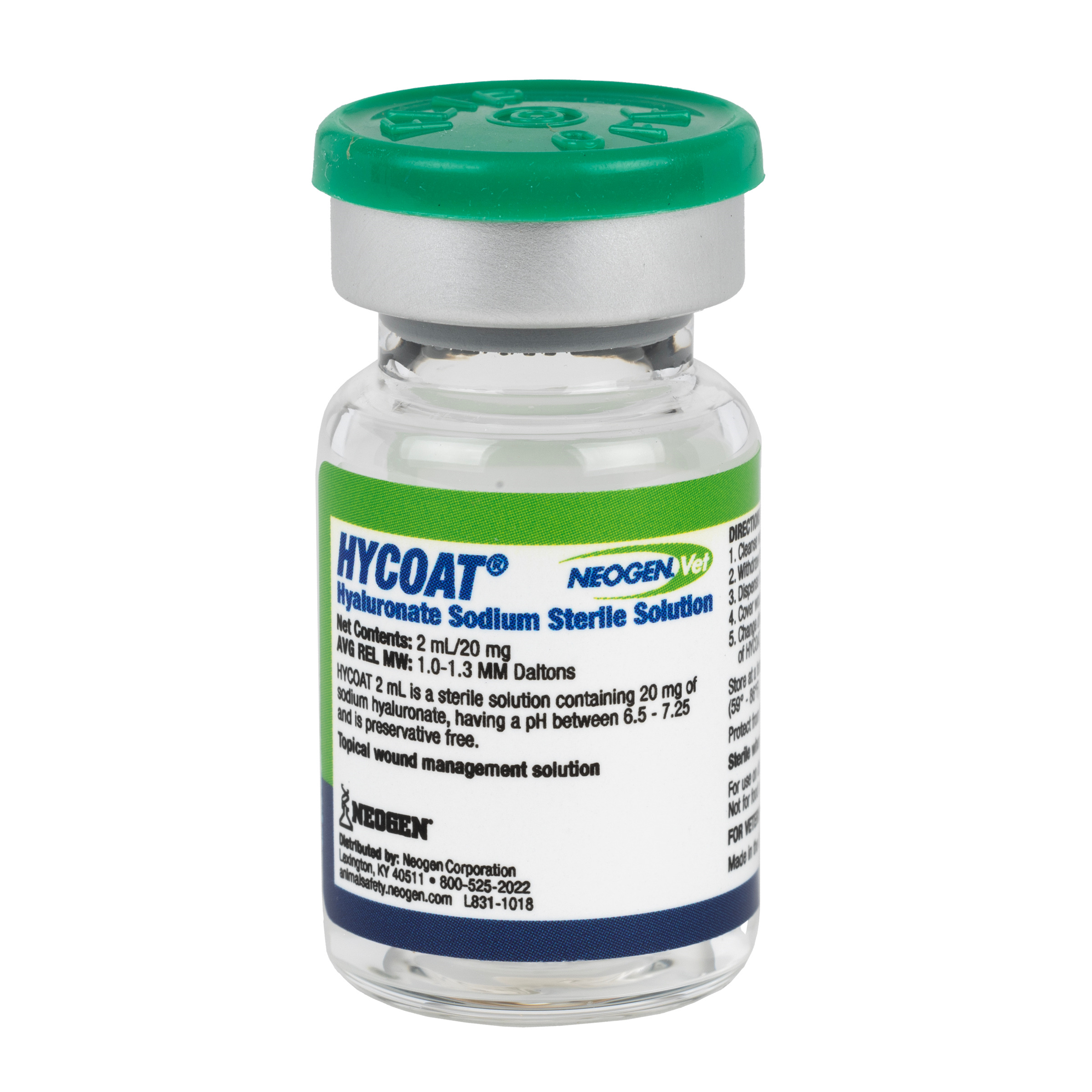
Neogen Vet HYCOAT® Hyaluronate Sodium Sterile Solution recalled due to Microbial Contamination, USA
3 weeks ago •source fda.gov
United States
Neogen Corporation (NASDAQ: NEOG) is voluntarily recalling all lots within expiry of Neogen®Vet HYCOAT® Hyaluronate Sodium Sterile Solution, for use in dogs, cats, and horses, to the veterinarian level. Neogen distributes this product, which is manufactured by a third-party supplier. Neogen received a number of reports of adverse events in horses following intra-articular injections of this product, which is inconsistent with its labeled, intended use. Neogen®Vet HYCOAT® Hyaluronate Sodium Sterile Solution was distributed nationwide to animal health distributors and veterinarians.While the company's investigation into this issue is ongoing, out of an abundance of caution, the 2mL/20mg product vials are also being recalled.
Risk Statement: This product is labeled as a sterile solution, but due to microbial contamination of certain lots, the recalled lots should no longer be considered sterile. Vials that contain a microbial contaminant potentially result in infection in the animal. The risk is particularly acute when used as an intra-articular injection (which is inconsistent with its labeled, intended use). There is also a risk if used according to the label as a topical wound management system in surgical wounds, burns, ulcers, and autograft procedures. To date, Neogen has not received reports of adverse events when used in a manner consistent with the labeled use.
The product is intended for use as a topical wound management system and is packaged in vials of 2mL/20 mg (UPC Code 726087089386) and 10mL/50mg (UPC Code 726087089393).
The subject product lots within expiry include the following:
2 mL/20 mg lot numbers: 0236735, 0236736, 0336746, 0336747, 0536760, 0536761, 0636768, 0636769, 0836792, 0836785, 0836788, 0836789, 0936794, 0936795, 1036801, 1036802, 1036803, 1036804, 1136807, 1136808, 0246837, 0246838, 4L001B, 4L002
10 mL/50 mg lot numbers: 0136731, 0736777, 0346843, 5A001
This recall has been initiated due to microbial contamination in certain lots of 10 mL/50 mg product vials.
Comments
Comment