Recall notice
Lactated Ringer’s and Sodium Chloride Injections recalled due to Particulate Matter, USA
4 months ago •source fda.gov
United States
B. Braun Medical Inc. (B. Braun) is voluntarily recalling two lots of Lactated Ringers Injection USP 1000 mL, and 0.9% Sodium Chloride Injection USP 1000 mL to the hospital level due to the presence of particulate matter inside the container. Product was distributed Worldwide and Nationwide in the United States to describe type of outlets (wholesale/retail/via internet).The product has a reasonable probability of causing pulmonary emboli (blockage in pulmonary blood vessels), occlusions of other blood vessels (which can lead to tissue death and possible organ damage), and/or phlebitis (inflammation of the walls of veins, which may lead to clotting). Systemically, foreign particles infused intravenously can cause systemic activation of the immune system, organ dysfunction, and hemolysis (breakdown of blood cells).
If the particulate matter is observed before use, a minor delay could occur while obtaining a replacement product. If the particulate matter is loose and the container is used on a patient, there is a potential for the particulate to be infused into the circulatory system. This could lead to patient harm that may require additional medical intervention and/or lead to permanent impairment or death.
The product is used as a state indication(s) and is packaged in state type of packaging, number of units, and any associated codes. The affected products are as follows:
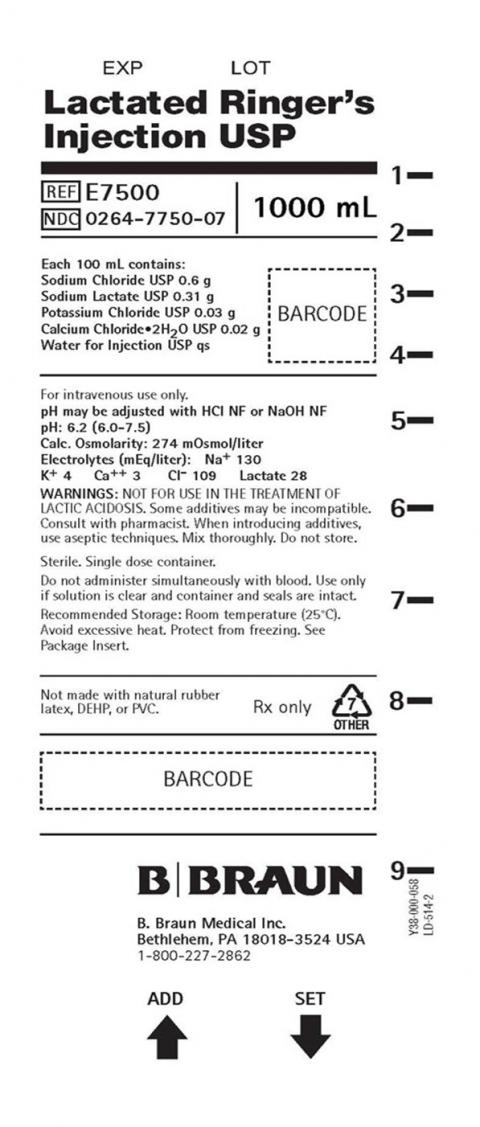
PRODUCT: Lactated Ringers Injection USP 1000 mL
- Product Catalog Number: E7500
- NDC Number: 0264-7750-07
- Size: 1000 mL
- Lot Number: J4S807
- Distribution Range: US
- Expiration Date: 31 MAY 2027 (Manufactured: 26 DEC 2024 – 10 APR 2025)
PRODUCT: 0.9% Sodium Chloride Injection USP 1000 mL
- Product Catalog Number: E8000
- NDC Number: 0264-7800-09
- Size: 1000 mL
- Lot Number: V3K770
- Distribution Range: US
- Expiration Date: 31 JAN 2026 (Manufactured: 15 NOV 2023 – 25 SEP 2024)
B. Braun has identified through complaints the potential for the product to contain particulate matter in solution. This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/b-braun-medical-issues-voluntary-nationwide-recall-lactated-ringers-injection-usp-1000-ml-and-09
Comments
Comment