Recall notice
Infusion Pump Thermal Damage Issue from Smiths Medical, United States
7 months ago •source fda.gov
United States
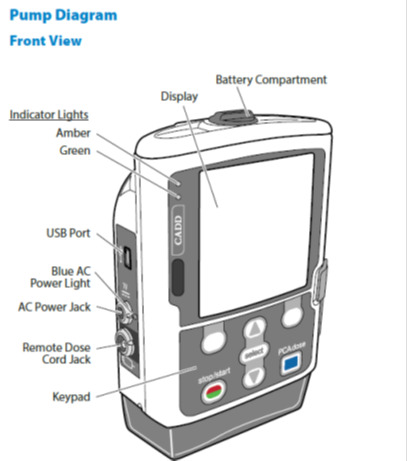
Smiths Medical has issued a recall for certain CADD-Solis Ambulatory Infusion Pumps due to potential thermal damage issues. The recall affects devices distributed for use in hospital and homecare settings. The FDA has classified this as a serious recall.The recall was initiated after Smiths Medical identified issues with the rechargeable battery pack and Wireless Communication Module, which could lead to thermal damage.
Smiths Medical has identified three safety concerns involving potential overheating in certain infusion pumps:
- Battery Pack Circuit Board Damage: Internal damage to the circuit board inside the rechargeable battery pack could cause the plastic casing to melt, especially on the top and bottom surfaces.
- Wireless Module Battery Overheating: Malfunctions in the Wireless Communication Module’s circuit board might lead to the battery housing melting due to overheating.
- Battery Compartment Issues: Battery separators that are broken or out of place, or the presence of debris in the battery area, could cause electrical shorts by connecting the battery contacts improperly.
These problems can result in the battery failing or becoming unsafe, possibly delaying or interrupting treatment. Depending on the medication and clinical setting, this could lead to severe patient harm or even death. Affected devices would typically trigger standard “Low Battery” or “Battery Depleted” alerts. In some cases, excess heat could be produced, posing a risk of burns or thermal injuries.
AFFECTED PRODUCT:
The affected products include the CADD-Solis Ambulatory Infusion Pump and CADD-Solis VIP Ambulatory Infusion Pump. Specific reference and serial numbers are impacted. The recall involves updating use instructions rather than removing the devices.
If you or a loved one are harmed or experiencing any symptoms, it is important to report it. Reporting can help to detect & resolve outbreaks early and prevent others from being harmed, and enables better surveillance. If symptoms persist, seek medical care.
Source: www.fda.gov/medical-devices/medical-device-recalls/infusion-pump-recall-infusion-pump-thermal-damage-issue-smiths-medical
23
Comments
Comment