Recall notice
Cyclobenzaprine Hydrochloride Tablets recalled due to Mislabeling, USA
5 months ago •source fda.gov
United States
East Brunswick, NJ, Unichem Pharmaceuticals (USA), Inc. is voluntarily recalling one (1) lot of Cyclobenzaprine Hydrochloride Tablets USP 10 mg, to the consumer level. The Cyclobenzaprine 10mg (90ct) label was inadvertently placed on a bottle containing Meloxicam 7.5 mg tablets. The product was distributed nationwide in the United States to distributors, and further downstream distribution occurred to retailers and subsequently consumers.Meloxicam Tablets USP, 7.5 mg is a non-steroidal anti-inflammatory drug, indicated for use in Osteoarthritis, Rheumatoid Arthritis, and Juvenile Rheumatoid Arthritis. Meloxicam Tablets, USP, 7.5 mg are light yellow, round, flat beveled edged, tablets with “U & L” debossed on one side and “7.5” debossed centrally on the other side.
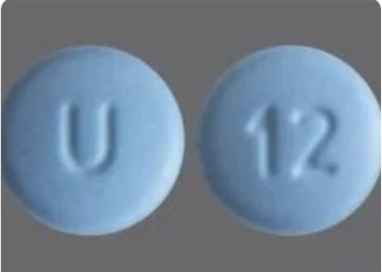
Cyclobenzaprine Hydrochloride Tablets USP, 10mg is a muscle relaxer and indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Cyclobenzaprine Hydrochloride Tablets, USP, 10 mg, are blue colored, film-coated, round-shaped, biconvex tablets, debossed with “U” on one side and “12” debossed on the other side.
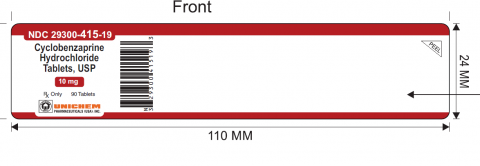
The mislabeled bottles of Cyclobenzaprine Hydrochloride Tablets USP, 10mg, but containing Meloxicam 7.5mg tablets, can be identified by the lot number GMML24026A and expiry of Sept 2027 and NDC 29300-415-19 printed on the label of the 90-count bottles.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/unichem-pharmaceuticals-usa-inc-issues-voluntary-nationwide-recall-cyclobenzaprine-hydrochloride
Comments
Comment