Report by
Safety Report: Flowflex™ SARS-CoV-2 Antigen Rapid Test (Self-Testing) - recalled due to Unauthorized U.S. distribution, USA
2 years ago •source fda.gov
Recall notice
United States
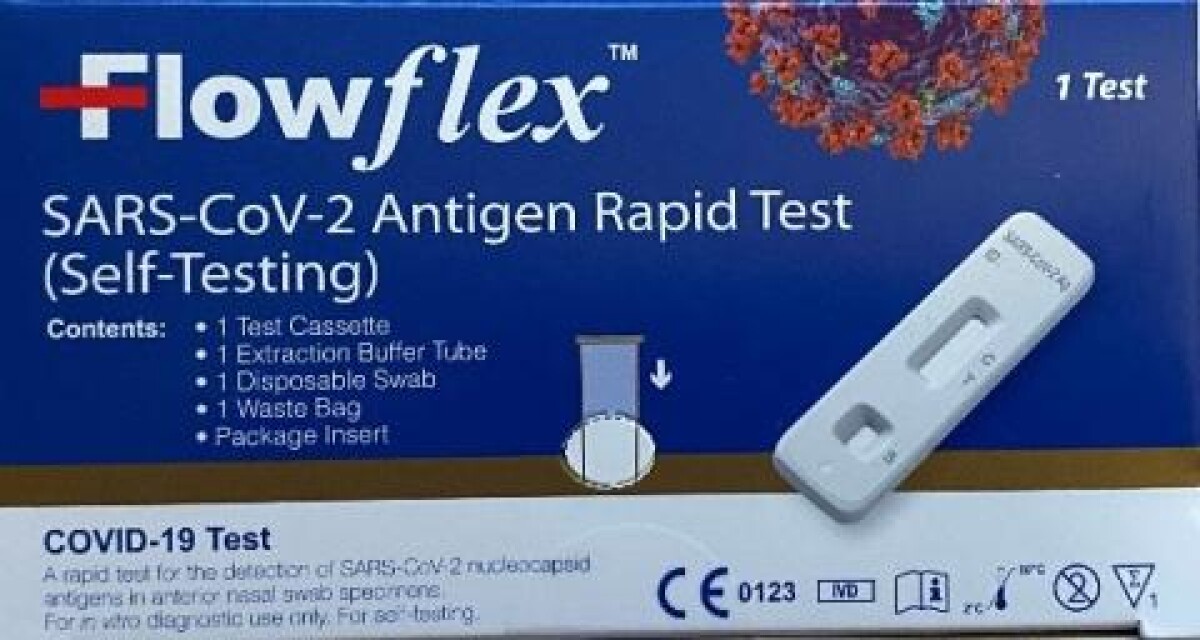
ACON Laboratories, Inc. (“ACON Laboratories”), the legal manufacturer of the “Flowflex™ COVID-19 Antigen Home Test” (FDA Emergency Use Authorization EUA210494), has identified the U.S. distribution of unauthorized, adulterated and misbranded counterfeit product having the trade name “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing).” ACON Laboratories is not importing the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” into the U.S. as it is only authorized for sale in Europe and other markets, under the CE mark. This press release serves as public announcement that this CE marked product is being recalled from the U.S. market.The “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” cannot be legally imported, distributed, or used in the U.S. market as it has not been approved, cleared, or authorized by the FDA. The “Flowflex COVID-19 Antigen Home Test” cannot be legally imported, distributed, or used in the European market as it is not CE marked. These two products have been authorized by the U.S. FDA and registered under CE Mark authorities separately under different product registration requirements, and therefore:
- This recall shall have no impact on the distribution and use of the CE marked “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” in Europe and other markets outside of the U.S.
- This recall shall have no impact on the distribution and use of the FDA authorized “Flowflex COVID-19 Antigen Home Test” in the United States.
Risk Statement: COVID-19 Antigen tests in the U.S. market that lack FDA approval, clearance, or authorization can pose significant risk since they may lead to inaccurate test results, including false negative or false positive test results. COVID-19 Antigen tests in Europe without the CE mark can pose significant risk since they may lead to inaccurate test results, including false negative or false positive test results.
False-negative antigen test results may lead to delayed diagnosis or inappropriate treatment of SARS-CoV-2, which may cause people harm including serious illness and death. False-negative results can also lead to further spread of the SARS-CoV-2 virus, including when people are grouped into cohorts (that is, they are housed together) in health care, long-term care, and other facilities based on these false test results.
Actions to limit exposure based on false-negative results might not be taken, such as isolating people, limiting contact with family and friends, and limiting ability to work. False-positive antigen test results may lead to a delay in both the correct diagnosis and the initiation of an appropriate treatment for the actual cause of a person’s illness, which could be another life-threatening disease that is not COVID-19. False-positive results could also lead to further spread of the SARS-CoV-2 virus when presumed positive people are grouped into cohorts (that is, they are housed together).
To date, ACON Laboratories has not received any reports of adverse events related to the products addressed in this public press release and is issuing this recall out of an abundance of caution.
If you have received the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” with the blue box in the U.S. market, you should stop using this product and dispose of it. The product has not been approved, cleared, or authorized for use in the U.S. To help differentiate the recalled product from the FDA authorized product, please find the table below, highlighting differences in the product kit box labeling. Note that the CE marked product has “ACON Biotech (Hangzhou) Co., Ltd.” as the manufacturer in place of “ACON Laboratories, Inc.”
- Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) | Not FDA Authorized (Recalled Product)
- Flowflex COVID-19 Antigen Home Test | FDA Authorized
Please see the pictures of the products below. ACON is working closely with the FDA and other law enforcement agencies to ensure that only the FDA authorized “Flowflex COVID-19 Antigen Home Test” is distributed in the U.S. Any distribution of the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” should be reported immediately to ACON Laboratories and the FDA at the numbers provided below.
Company name: ACON Laboratories, Inc.
Brand name: Flowflex™
Product recalled: SARS-CoV-2 Antigen Rapid Test (Self-Testing)
Reason of the recall: Unauthorized U.S. distribution-counterfeited product
FDA Recall date: March 11, 2022
Check the full recall details on www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/acon-laboratories-issues-recall-non-eua-authorized-flowflextm-sars-cov-2-antigen-rapid-test-self
Source: FDA
312
1 Share