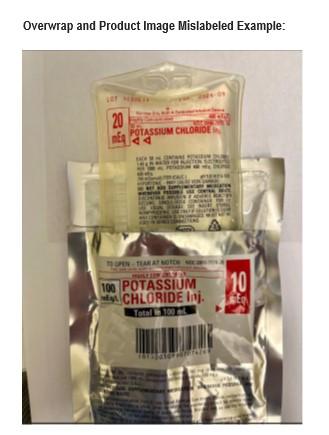
Recall notice
氯化钾注射液因氯化钾过量而召回, USA
3个月前 •source fda.gov
United States
Otsuka ICU Medical LLC is issuing a voluntary recall to the user level, for a MISLABELLED lot of POTASSIUM CHLORIDE Inj. 20 mEq, NDC 0990-7077-14. The OVERWRAP label of lot 1030613, Expiration Date: 09-30-2026 may incorrectly identify the product as POTASSIUM CHLORIDE Inj. 10 mEq with NDC 0990-7074-26. Otsuka ICU Medical LLC has identified this discrepancy due to a manufacturing issue. The dosage is correctly printed on the labeling affixed to the product bag which is not visible when the 10 mEq OVERWRAP is in place. This notification details the issue and the required steps for you to perform. The affected product lot was manufactured on 15 April 2025 and distributed in the United States between 23 May 2025 through 26 August 2025.If the incorrect dosage on the 10 mEq overwrap is used instead of the correct 20mEq dosage printed on the product, an overdose of potassium chloride is possible. Overdose of potassium chloride can lead to hyperkalemia. Hazards of severe hyperkalemia after large intravenous overdoses causes neuromuscular dysfunction including muscle weakness, ascending paralysis, listlessness, vertigo, mental confusion, hypotension, cardiac dysrhythmias, or death from cardiac arrest. Premature infants, patients on chronic parenteral nutrition, patients who have a history of cardiac arrythmias, patients with chronic renal insufficiency, patients who have acute renal failure, patients on potassium-sparing diuretics—all are at risk for adverse and potentially fatal outcomes. Otsuka ICU Medical LLC has not received reports of adverse events associated with this issue to date.
The affected product lot (Located on the top left of the product bag or the case label is:
- NDC NUMBER: 0990-7077-14
- LIST NUMBER: 070770452
- PRODUCT: Potassium Chloride Injection 20 mEq
- LOT NUMBER: 1030613
- EXPIRATION DATE: September 30, 2026
- CONFIGURATION: 50 mL in Flexible Container
- NDC NUMBER: 0990-7074-26
- LIST NUMBER: 070740452
- PRODUCT: Potassium Chloride Injection 10 mEq
- LOT NUMBER: N/A
- EXPIRATION DATE: N/A
- CONFIGURATION: 100 mL in Flexible Container
DESCRIPTION OF CASES BEING RECALLED:
- NDC NUMBER: 0990-7077-14
- BARCODE NUMBER: (01)20309907077141
- LOT NUMBER: 1030613
- EXPIRATION DATE: September 30, 2026
- CONFIGURATION: 24 per case
The U.S. Food and Drug Administration (FDA) has been notified of this action.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/otsuka-icu-medical-llc-issues-voluntary-nationwide-recall-20-meq-potassium-chloride-injection-due
评论
评论