Recall notice
Prazosin Hydrochloride, Capsules recalled due to CGMP deviations, United States
5 months ago •source accessdata.fda.gov
United States
Teva Pharmaceuticals USA, Inc. has initiated a voluntary recall of Prazosin Hydrochloride capsules due to CGMP deviations. The recall affects products distributed nationwide in the U.S. due to test results showing impurity levels above acceptable limits. A total of 580,844 bottles are affected.AFFECTED PRODUCTS:
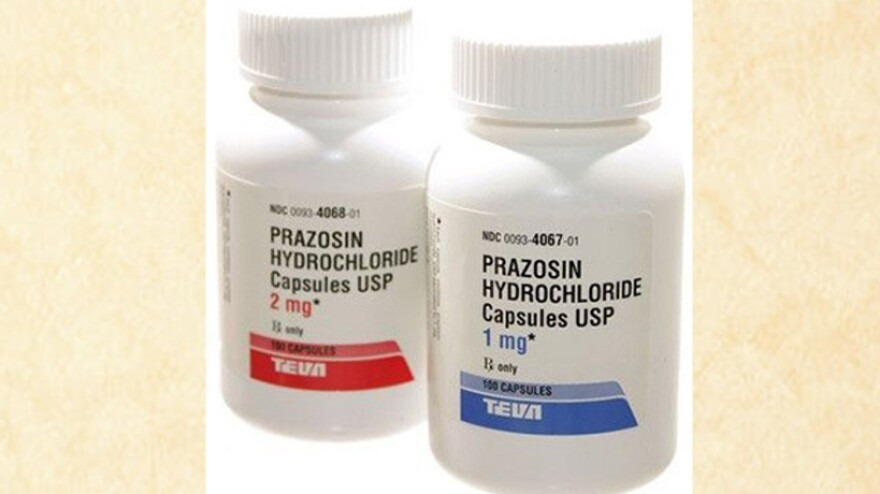
PRODUCT: Prazosin Hydrochloride Capsules, USP, 1 mg (Rx only)
- Size: 100 Capsules / 1000 Capsules
- NDC: 0093-4067-01 / 0093-4067-10
- Lot: 3010544A, 3010545A
- Exp. Date: 10/2025
- Product Quantity: 181,659 bottles
- Distributed by TEVA Pharmaceuticals USA, Inc.
PRODUCT: Prazosin Hydrochloride Capsules, USP, 2 mg (Rx only)
- Size: 100 Capsules / 1000 Capsules
- NDC: 0093-4068-01 / 0093-4068-10
- Lot: 3010398A, 3010399A, 3010400A, 3010401A, 3010353A, 3010439A, 3010388A, 3010526A, 3010527A, 3010591A, 3010343A, 3010352A, 3010468A, 3010469A, 3010461A
- Exp. Date: 10/2025 – 07/2026
- Product Quantity: 291,512 bottles
- Distributed by TEVA Pharmaceuticals USA, Inc.
PRODUCT: Prazosin Hydrochloride Capsules, USP, 5 mg (Rx only)
- Size: 100 Capsules / 250 Capsules / 500 Capsules
- NDC: 0093-4069-01 / 0093-4069-52 / 0093-4069-05
- Lot: 3010403A, 3010385A, 3010404A, 3010405A, 3010510A, 3010528A, 3010354A, 3010592A, 3010605A, 3010611A, 3010612A
- Exp. Date: 02/2026 – 03/2026
- Product Quantity: 107,673 bottles
- Distributed by TEVA Pharmaceuticals USA, Inc.
Reason for recall:
CGMP Deviations-Test results for N-nitroso Prazosin impurity C that are above the Carcinogenic Potency Categorization Approach (CPCA) acceptable intake limit for the above-specified lots.
The issue was identified through test results indicating N-nitroso Prazosin impurity C levels above the Carcinogenic Potency Categorization Approach limits. The recall, classified as Class II, was initiated on October 7, 2025, and classified on October 24, 2025.
Source: www.accessdata.fda.gov/scripts/ires/
23
Comments
Comment