Recall notice
AVKARE Eye Drops Recalled Over Sterility Issues, United States
9 months ago •source avkare.com
United States
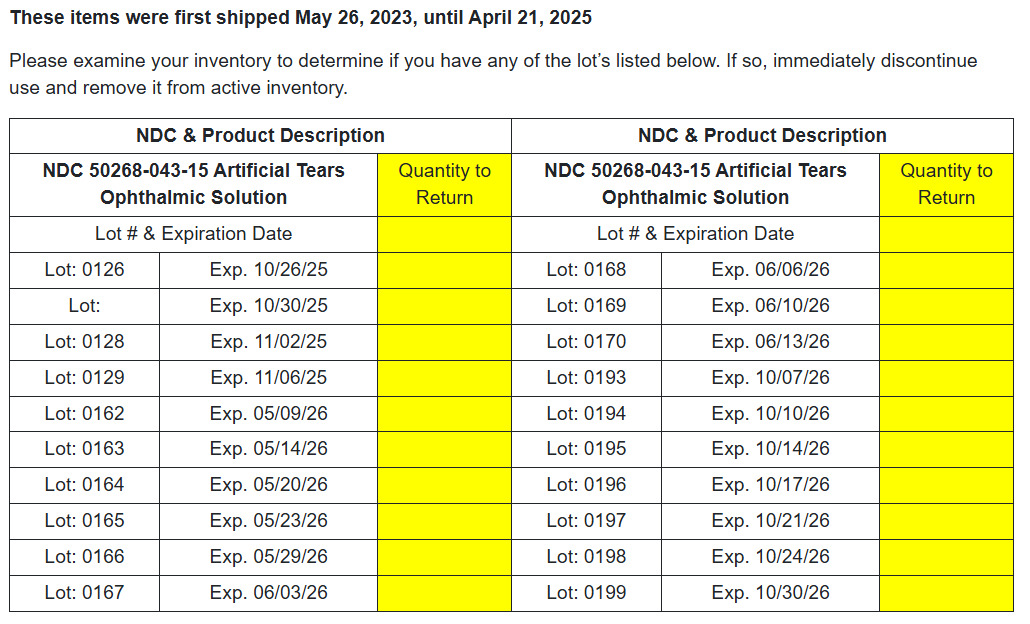
BRS Analytical Service, LLC. has initiated a voluntary recall of various ophthalmic products due to manufacturing deviations identified during an FDA audit. The potential health hazard to users is currently unknown, but the deviations may lead to products of unacceptable quality. The recall was initiated after the FDA identified manufacturing deviations during an audit. The potential risk to patients from using these products cannot be ruled out. The recall is being carried out with the knowledge of the US Food and Drug Administration. The products were shipped nationally between May 26, 2023, and April 21, 2025. This has changed to the retail level only.AFFECTED PRODUCTS
NDC# 50268-043-15 Artificial Tears Ophthalmic Solution
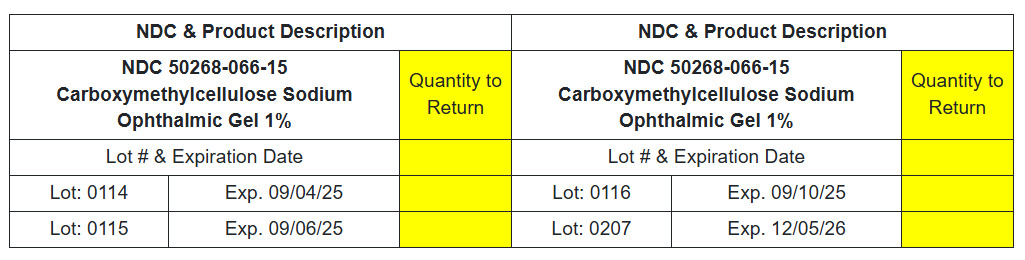
NDC# 50268-066-15 Carboxymethylcellulose Sodium Ophthalmic Gel 1%
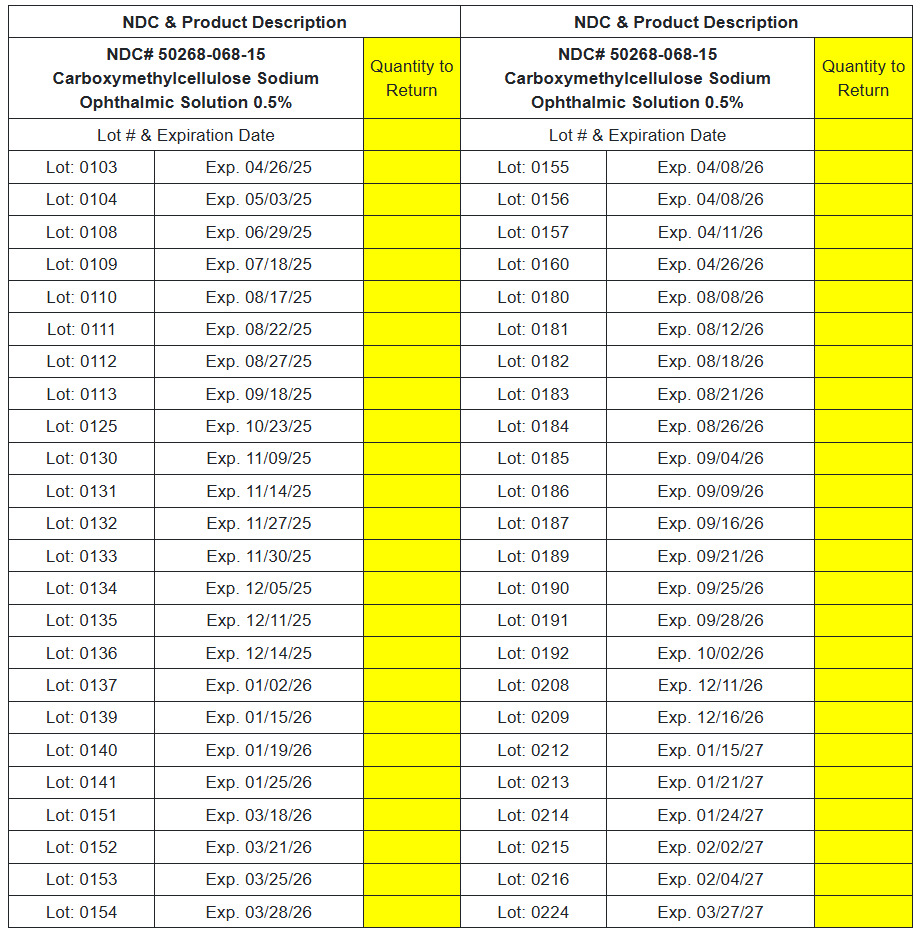
NDC# 50268-068-15 Carboxymethylcellulose Sodium Ophthalmic Solution
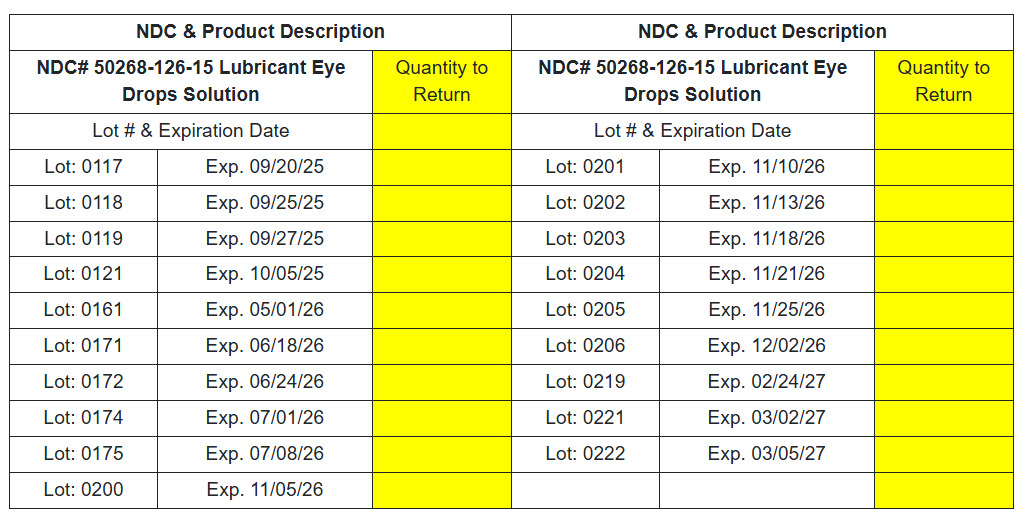
NDC# 50268-126-15 Lubricant Eye Drops Solution
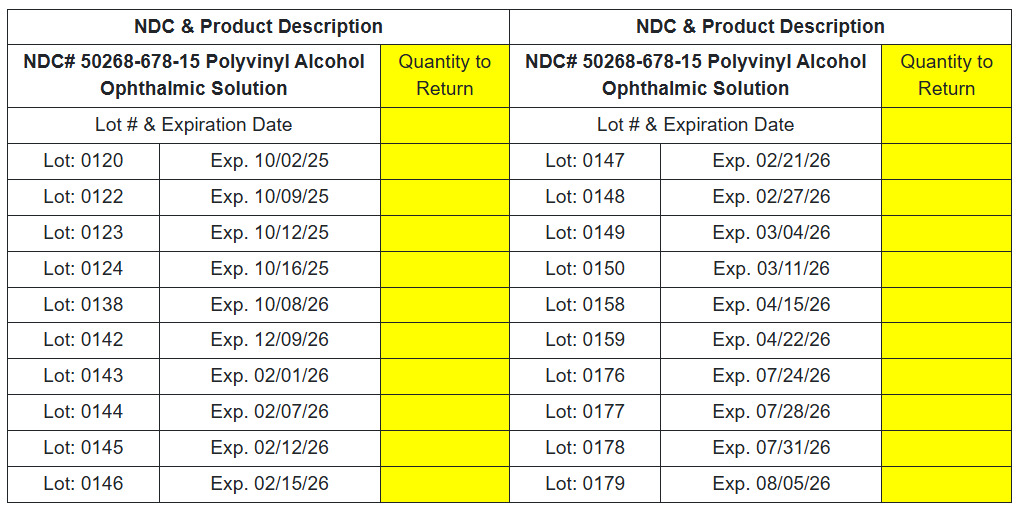
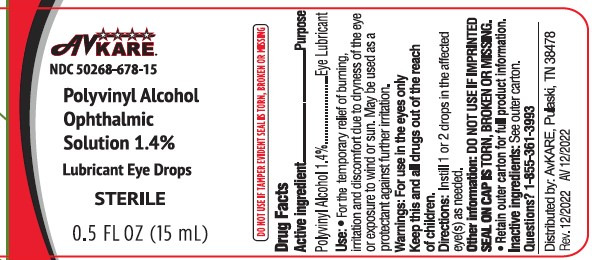
NDC# 50268-678-15 Polyvinyl Alcohol Ophthalmic Solution
You can check the affected product details at the link below.
Consumers are advised not to use the product and to return it to where they purchased it.
If you or a loved one is harmed or experiencing any symptoms, it is important to report it. Reporting can help to detect & resolve outbreaks early and prevent others from being harmed, and enables better surveillance. If symptoms persist, seek medical care.
Source: www.avkare.com/recall
22
Comments
Comment