Recall notice
Sensodyne PRONAMEL toothpaste recalled due to Mislabeling, United States
4 months ago •source accessdata.fda.gov
United States
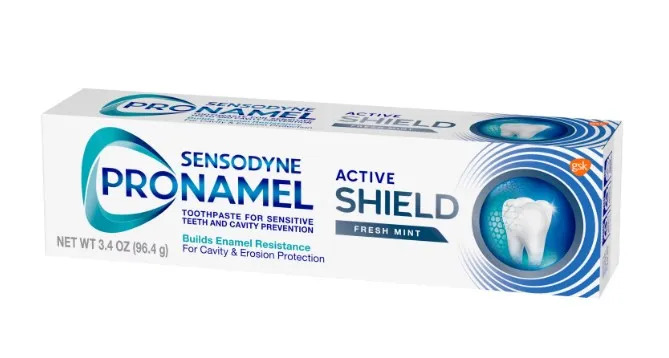
Haleon US Holdings LLC has initiated a voluntary recall of Sensodyne PRONAMEL Active SHIELD Toothpaste for Sensitive Teeth due to a labeling mix-up. The product, distributed nationwide in the USA, has mismatched labels on the outer carton and tube, though the toothpaste inside is correctly labeled.AFFECTED PRODUCT:
- Sensodyne PRONAMEL (Potassium nitrate 5%, Sodium fluoride 0.25%) Active SHIELD Toothpaste, Fresh Mint
- In a 3.4 oz (96.4g) tube.
- It is packaged in cases of 6 tubes x 2 inners
- With a UPC code of 3 10158 35691 2.
- The affected lot number is 5058RB
- With an expiration date of 08/31/2027
- Totaling 46,692 tubes.
The labeling issue was identified through internal checks, leading to the recall classified as Class III. The recall was initiated on August 5, 2025, and classified on August 19, 2025.
Source: www.accessdata.fda.gov/scripts/ires/
22
Comments
Comment