Report by
Information About Coronavirus Covid-19
The current Coronavirus disease 2019 (Covid-19) pandemic is caused by the novel coronavirus strain called 2019-nCoV that is thought to have originated in live food markets in Wuhan, China through human-animal contact. The coronavirus family includes viruses that caused SARS and MERS.
How is it transmitted?
Coronaviruses strains usually cause respiratory infections and are typically transmitted by inhalation of the virus through the mouth or nose. People become infected by inhaling virus particles through the air or by coming in contact with contaminated surfaces or other infected people. Studies have shown that the virus is able to survive on surfaces for up to 48 hours and live virus has been found in Covid-19 patients' fecal matter. But the virus is killed by heat and disinfectants.
| Survival Time | Surface |
|---|---|
| 96 hours | glass surfaces |
| 72 hours | plastic |
| 72 hours | stainless steel |
| 4 hours | copper |
| 24 hours | cardboard |
| 3 hours | aerosols |
| 1-2 days | fecal matter |
| 4 days | diarrhea |
Who is at risk?
While most people think only the elderly and immunocompromised are at risk, there is new data suggesting that adults under 54 and children are at risk for serious illness as well. The mortality rate of the virus is currently thought to be between 1-4% but this is highly dependent on age and preexisting conditions.
To read more about the probability of death, click here
Signs of infection?

Common signs of infection include respiratory symptoms such as fever, cough, shortness of breath and breathing difficulties. In more severe cases including those with weakened immune systems, an infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death.
How to Stop Coronavirus?
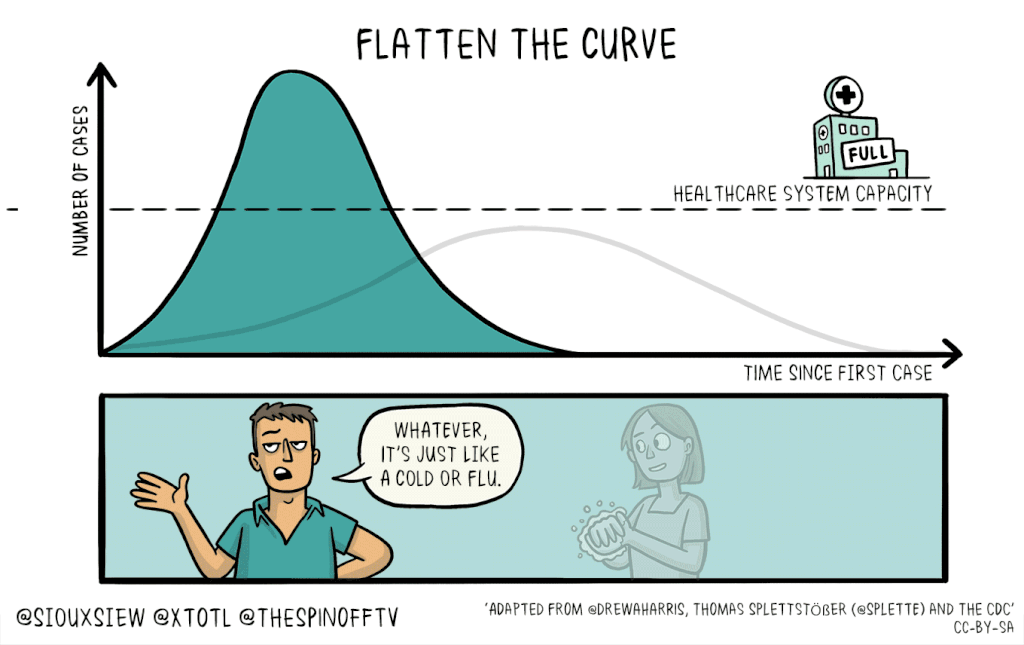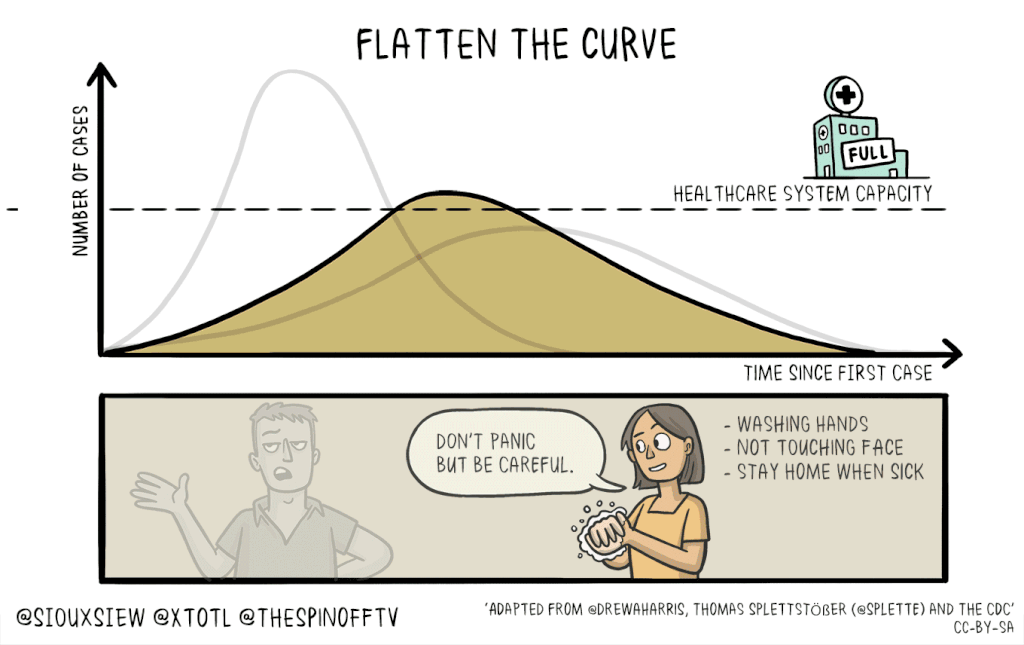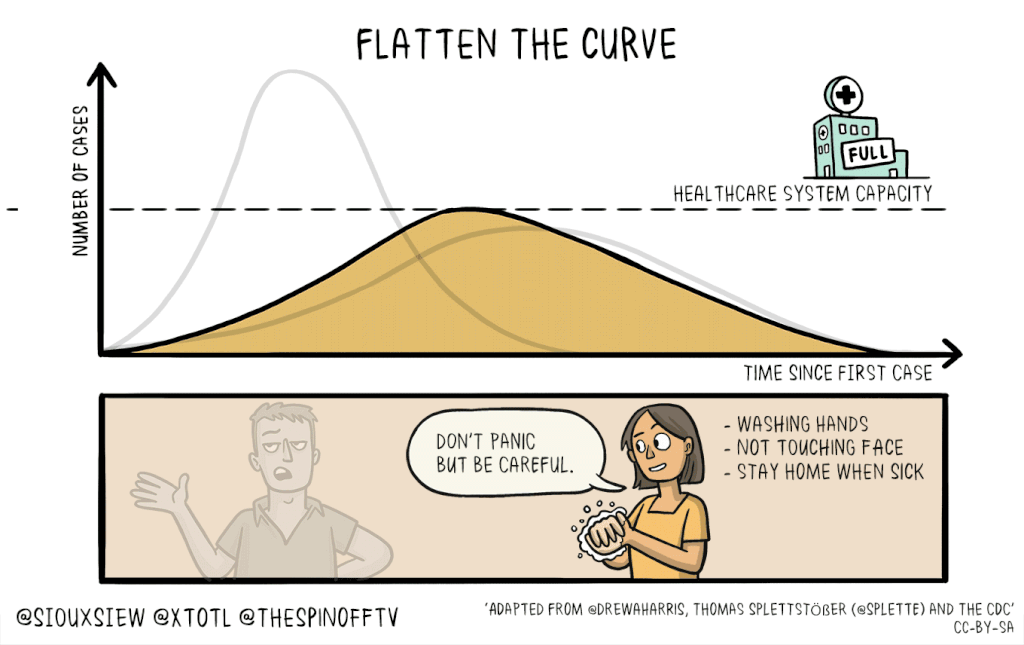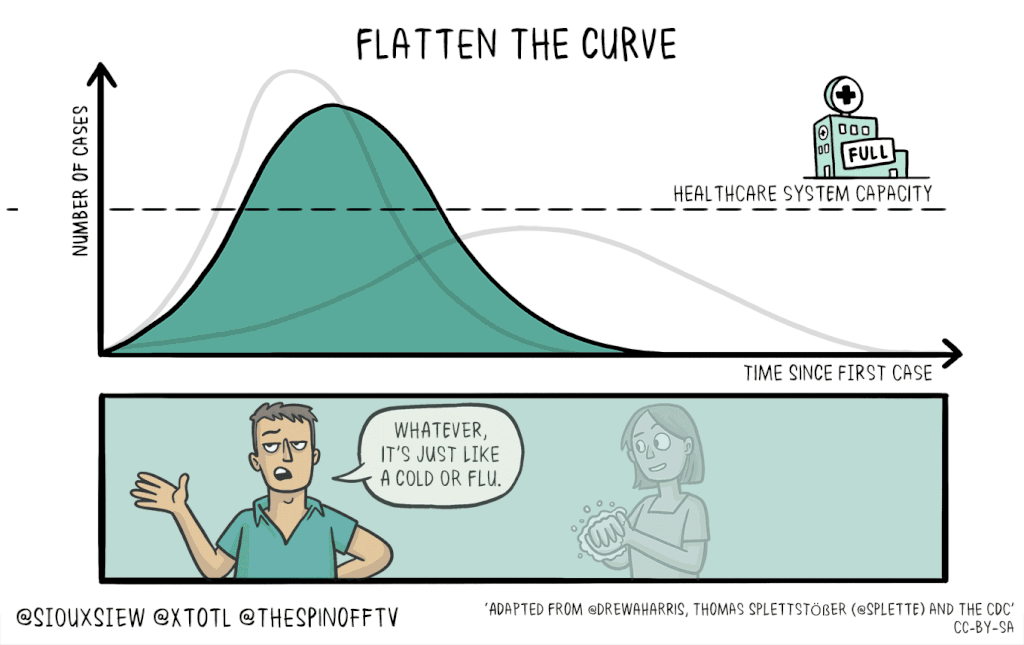
There is no known cure or vaccine for the current coronavirus Covid-19 pandemic, so the best technique to control the spread is social distancing: Keeping people home as much as possible. This will help flatten the curve of infection, which means reducing the spread of infection to create a flatter peak to avoid overwhelming resources.
To listen to people in communities around the world, click on the links below:
People in the United States unable to get tested:
Sick man showing symptoms of Covid-19 in the United States that is unable to get tested
Situation Reports of what is happening around the world:
Restaurant Owner in Taipei, Taiwan
Safely HQ User in Lyon, France

