As a global community reporting platform focused on consumer wellbeing, we receive a wide range of reports. Since the launch of the Covid-19 vaccines we have accumulated reports relaying peoples’ experiences after receiving doses of the currently approved vaccines e.g. Moderna, Pfizer, Jansen, Johnson & Johnson, and Oxford-AstraZeneca. We took some time to analyze, and categorize the results so far, so we can share back to our community and others who may find this helpful.
We hope that by sharing this information, people will feel better informed on the range of possible outcomes as they move ahead with their vaccinations, and for those who have been vaccinated already, we hope the results offer some comfort in knowing that you are not alone in experiencing certain side effects.
A percentage breakdown of reported side effects:
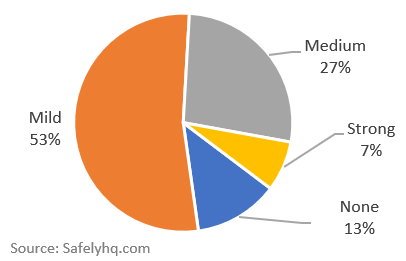
Percentage breakdown of Covid-19 Side Effects. Source: Safelyhq.com
To categorize reports we used the following approach:
- None: User stated they had ‘no side effects’
- Mild: Typically just soreness and side effects described as ‘mild’ by the users.
- Strong: Unusual or notable description, which includes trips to ER, or intense reactions.
- Medium: Everything that falls in between mild and strong.
Important: The focus of this story is the comparison between Moderna and Pfizer, please see our data notes at the bottom about reporting bias & data skew, we are not concluding that only 13% of vaccine recipients will escape side effects.
Side Effect Breakdown, Pfizer vs. Moderna

Covid-19 Side Effect Comparison — Pfizer vs Moderna. Source: Safelyhq.com
We more commonly saw reports with no side effects from Pfizer, and where Pfizer side effects were reported they were more commonly reported as mild vs Moderna.
What are the most commonly reported side effects?
Of people who experienced side effects, the most common mentions are shown below. We excluded soreness as this was common to the majority of reports.
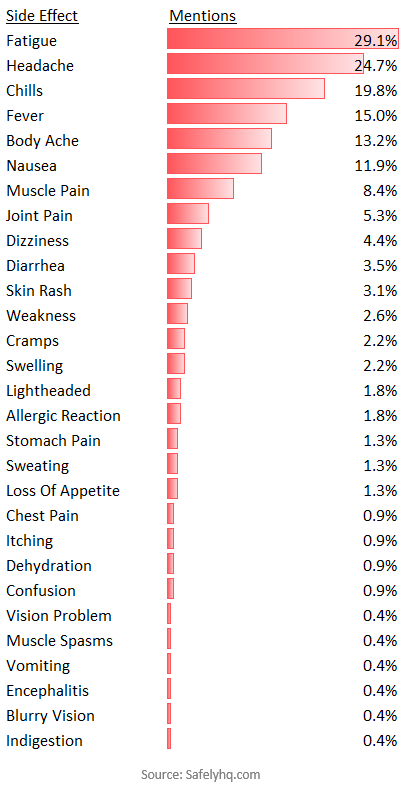
The Most Commonly Reported Covid-19 Vaccine Side Effects. Source: Safelyhq.com
What is the side effect breakdown: Moderna vs Pfizer?
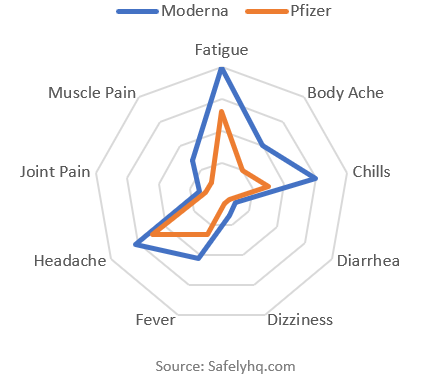
Top 10 Side Effect Mentions Comparing Pfizer & Moderna Source: Safelyhq.com
This shows the top 10 mentions by vaccine (again excluding soreness). Moderna has more mentions for each side effect. Moderna has a lot more side effect mentions per report — in total 60% higher than Pfizer.
What are some sample reports?
A sample ‘No Side Effects’ report:
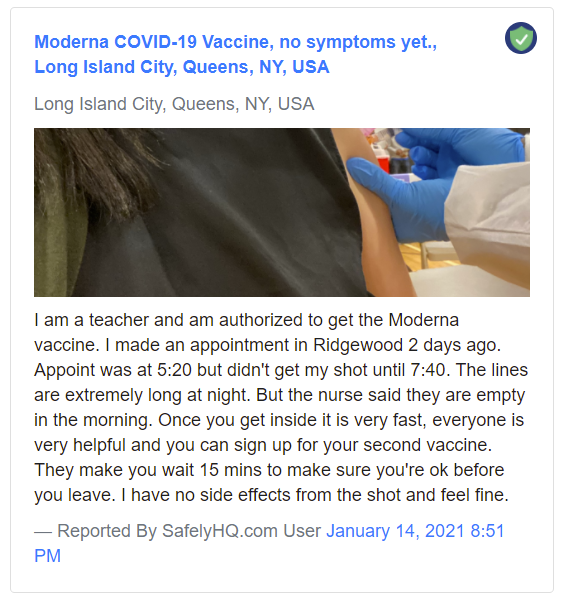
Sample report ‘No side effects’. Source: Safelyhq.com — Link
A sample ‘Mild’ report:
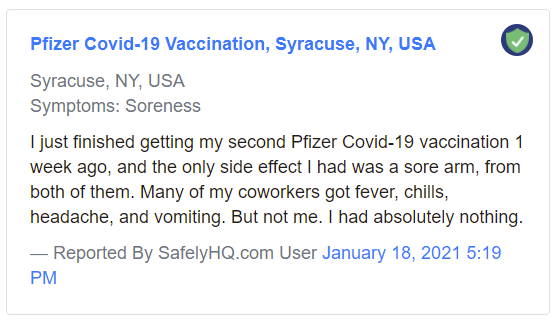
Sample ‘Mild’ report. Source: Safelyhq.com — Link
A sample ‘Medium’ Report:
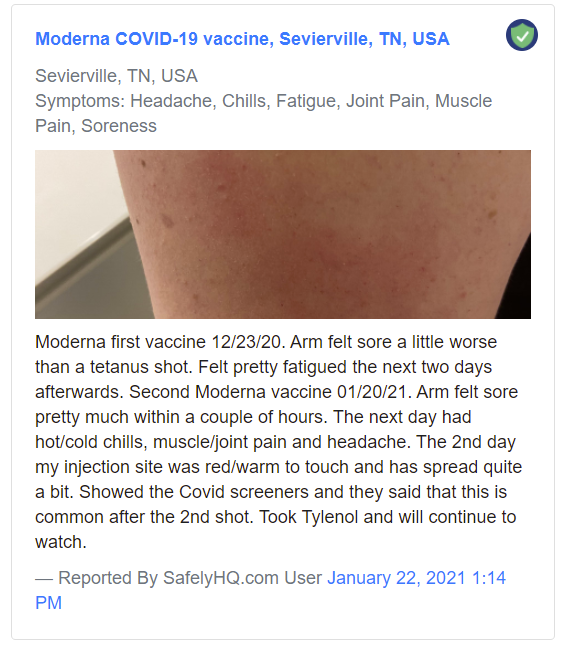
Sample ‘Medium’ report. Source: Safelyhq.com — Link
A sample ‘Strong Side Effects’ report:

Sample ‘Strong Side Effects’ report. Source: Safelyhq.com — Link
What can I do to help?
- Share your vaccine experience! If you had a vaccine, our community would love to see your report, as we continue to get reports, we will update and expand our analytics. To share you experience click here.
- Share this article! The more people are able to read other people’s experiences, the more we can help others on their vaccination journey.
How can I stay up to date with your work on Covid-19 Vaccines?
You are welcome to sign up to our free email list. Learn about new analysis , get the latest news, and get updates as new reports come in. To sign up click here.
What about Oxford, Johnson & Johnson and the other Vaccine Brands?
We have received reports for other vaccines but so far there is not enough data to include in this analysis. We would love you to share your experience if you had one of these vaccines. To share your experience click here.
Where can I see the reports and raw data?
You can view the reports on our covid-19 vaccine landing page here.
I got a Covid-19 vaccine, how can I participate and share my experience?
We welcome you to share your experience simply go to our covid-19 vaccine landing page and fill out the form as show below. To start click here
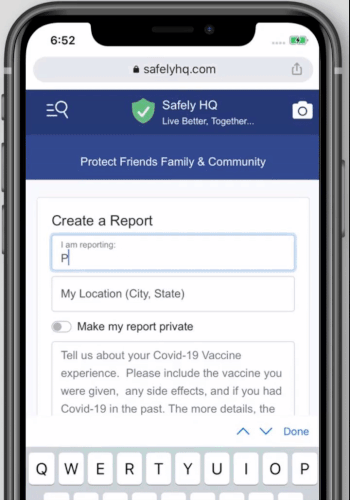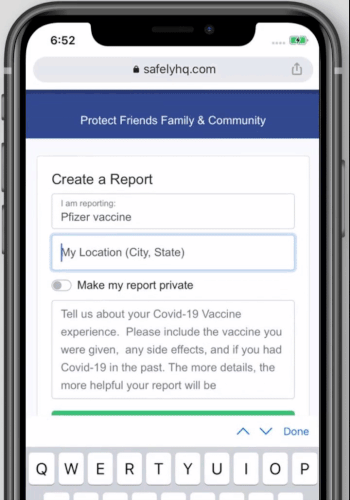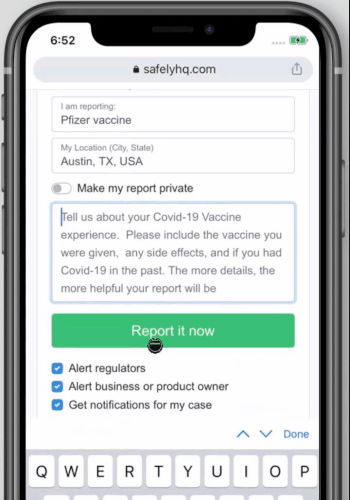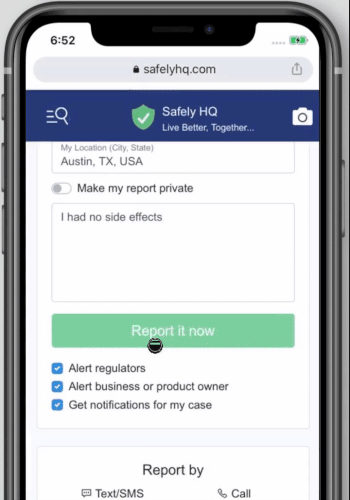
How to share your Covid-19 Vaccine experience on safelyhq.com
Which countries have we received reports from so far?
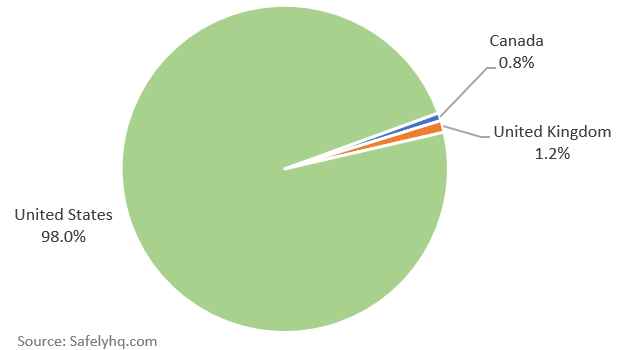
Covid-19 Vaccine Side Effects Reports by Country. Source: Safelyhq.com
Which states within the US participated so far?
We have received reports from most states in the US, with the highest participation coming from California, Texas, and Florida.
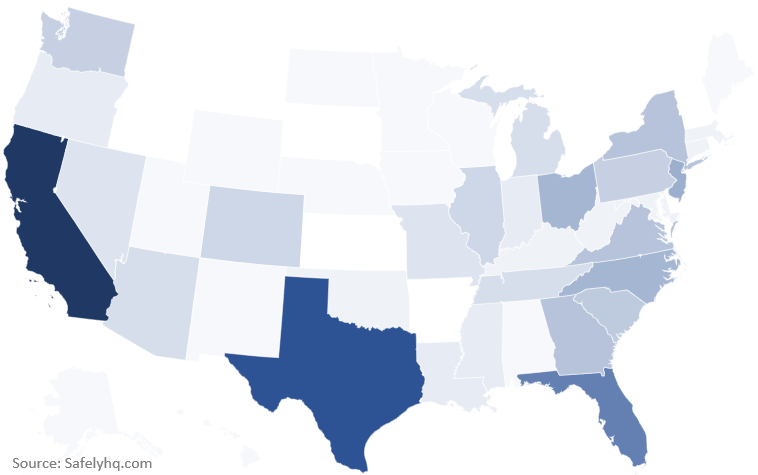
Source of US Covid-19 Vaccine Reports by State. Source: Safelyhq.com
Notes about the data
- Sample Size: This data represents a sample of 250 vaccine recipients, 125 Moderna, and 125 Pfizer, from the US, Canada, and the UK (primarily US) between January 1st 2021 and March 5th 2021.
- Data Skew: This data is likely skewed toward older recipients and/or higher risk populations as they have been the priority recipients of vaccinations so far in the US.
- Reporting Bias: To the extent that there is reporting bias whereby those people with side effects are more likely to share their experience than those without side effects — the overall ‘no side effects’ numbers may be under reported. However, all other things being equal, the relative comparison between Pfizer and Moderna may still reflect a fair comparison.






































