Brand name: Fresenius Kabi
Product recalled: Ketorolac Tromethamine Injection, USP, 30 mg/mL, and Ketorolac Tromethamine Injection, USP, 60 mg/2 mL
Reason of the recall: Presence of Particulate Matter
FDA Recall date: April 20, 2020
Recall details: Fresenius Kabi USA, LLC is …
Particulate matter was found in eight reserve sample vials. Administration of products containing particulate matter could obstruct blood vessels and result in local irritation of blood vessels, swelling at the site of injection, a mass of tissue that could become inflamed and infected, blood clots traveling to the lung, scarring of the lung tissues, and allergic reactions that could lead to life-threatening consequences.
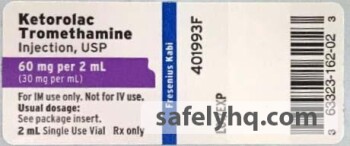
Ketorolac Tromethamine, a nonsteroidal anti-inflammatory drug, is indicated for the short-term (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level. The total combined duration of use of oral Ketorolac Tromethamine and Ketorolac Tromethamine injection should not exceed 5 days. Listed below is a table of the recalled lots distributed nationwide to wholesalers, distributors, hospitals, and pharmacies between May 5, 2018 and December 16, 2019, as well as a copy of the label:
- Ketorolac Tromethamine Injection, USP, 30 mg / mL, 1 mL fill in a 2 mL amber vial. NDC: 63323-162-01. Product code: 160201. (Check the recall notice for specific batch numbers)
- Ketorolac Tromethamine Injection, USP, 60 mg / 2 mL (30 mg / mL), 2 mL fill in a 2 mL amber vial. NDC: 63323-162-02. Product code: 160202. (Check the recall notice for specific batch numbers)
Check the full recalled product list and details on www.fda.gov
Source: FDA
Recent Interesting Reports
ForeverMen Natural Energy Boost - recalled due to undeclared allergens, USA
3 weeks ago •source www.fda.gov
Recall notice
Risk Statements: Men with diabetes, high blood pressure, high cholesterol, or heart disease, may be on medications that if taken with these products could lower blood pressure to dangerous levels that could be life threatening. The products affected are men with diabetes, high blood pressure, high cholesterol, or heart disease.
The product is marketed as dietary supplements for male sexual enhancement and is packaged in a blister card. 10 count box. We are notifying the public through this public announcement due to lack of ability to identify customers who may have received the product. FAonline INC. is notifying its customers that have the ForeverMen products to stop use and properly discard the product.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
In case you experienced harm from allergens or undeclared ingredients, it is important to report it. It can help to detect & resolve issues and prevent others from being harmed, and it enables better surveillance. If symptoms persist, seek medical care.
Company name: FAonline Inc
Brand name: ForeverMen
Product recalled: Natural Energy Boost
Reason of the recall: Product is tainted with sildenafil and tadalafil
FDA Recall date: April 02, 2024
Source: www.fda.gov
Self-adjusting lens adaptive glasses, 192-01 Northern Blvd, Flushing, NY 11354, USA
3 weeks ago •reported by user-xgfpb263
Empty Package, Levittown, PA, USA
2 weeks ago •reported by user-ydpht521
Unordered Package, Spokane, WA, USA
3 days ago •reported by user-fhxqx637
Unwanted package. I did not order this. I was sent a leg brace., Toms River, NJ, USA
3 weeks ago •reported by user-khrvy159
Received package - used. Bra - did not order, Mechanicsville, VA, USA
1 week ago •reported by user-mnqcp927
Delivery without an order submitted, Papillion, NE, USA
3 days ago •reported by user-fwzrg968
Planter scam, Libby, Montana, USA
2 weeks ago •reported by user-cfdg6856
Subgenix Bioribose I DID NOT BUY!, Smyrna, GA, USA
2 weeks ago •reported by user-rjww7298
I received this and don’t know why, Gilroy, CA, USA
3 weeks ago •reported by user-zmqgk555
Report by
IMPORTANT - YOUR REPORT IS QUEUED - IT MAY TAKE UP TO 12 HOURS FOR YOUR REPORT TO SHOW ON OUR HOME PAGE (IF NOT OPTED AS PRIVATE)
Visit our learn pages for more helpful information or, email us: support@safelyhq.com