Source: Osha.gov | Receipt Date: 2020-11-19
Report by
Cross Contamination
Updated:
Source: Osha.gov | Receipt Date: 2020-11-19
Fresenius Kabi USA Dexmedetomidine HCL in 0.9% Sodium Chloride Injection - recalled due to cross contamination with Lidocaine, USA
3 years ago •source www.fda.gov
Recall notice
Brand name: Fresenius Kabi USA
Product recalled: Dexmedetomidine HCL in 0.9% Sodium Chloride Injection
Reason of the recall: Cross Contamination with Lidocaine
FDA Recall date: November 19, 2020
Recall details: Fresenius Kabi USA is voluntarily recalling a single lot of Dexmedetomidine HCl
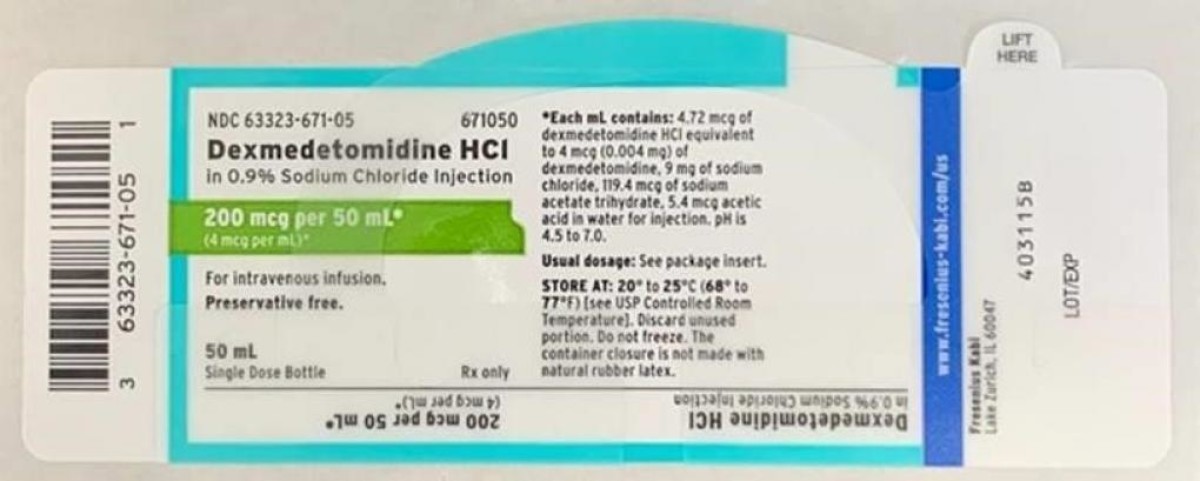
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection is approved for intravenous use and indicated for sedation of non-intubated patients prior to and/or during surgical and other procedures. Listed below is a table of the recalled lot distributed nationwide to wholesalers, distributors, hospitals and pharmacies between April 9, 2020 and April 13, 2020. An image of the label is also included below.
- Dexmedetomidine HCl in 0.9%. Sodium Chloride Injection, 200 mcg / 50 mL (4 mcg / mL), 50 mL fill in a 50 mL vial. NDC Number: 63323-671-50. Product Code: 671050. Batch number: 6123925. Expiration Date: 03/2022. First Ship Date: 04/09/2020. Last Ship Date: 04/13/2020
Fresenius Kabi is notifying its distributors and customers by letter and asking them to check their stock immediately and to quarantine and discontinue the use and distribution of any affected product.
Distributors should notify their customers and direct them to quarantine and discontinue distributing or dispensing any affected lots, and to return the product to Fresenius Kabi.
Customers with questions regarding this recall may contact Fresenius Kabi at 1-866-716-2459 Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. Central Time. Consumers should contact their physician or health care provider if they have experienced any problems that may be related to taking or using this drug product.
Check the full recall details on www.fda.gov
Source: FDA
Recent Interesting Reports
I received a jar of Verti Bioribose, Nellis Air Force Base, NV, USA
1 week ago •reported by user-pvbrz262
All I know is I did not order this. I don't know what it is.
I Google the name and this site came up.
Then I read the complaints and they're similar to what
I did not order all this, Nebraska 2, Grand Island, NE, USA
2 weeks ago •reported by user-dpxw9319
Faults advertising, Columbus, OH, USA
3 weeks ago •reported by user-dbyfd382
Unordered package from “fullfilment house”, Oklahoma City, OK, USA
4 weeks ago •reported by user-pzzkq495
Tampa, FL 33675-5708
Scam Heathers MOTTY, United States
3 weeks ago •reported by user-mfmbd859
Unordered Subgenix, Tampa, FL 33675, USA
4 weeks ago •reported by user-wwqg3798
Fullfillment House PO BOX 5708 Tampa FL 33675-5708
Verti products, Tampa FL. 33626
3 weeks ago •reported by user-dtwk5977
I recieved a bottle of subgenix bioribrose i did not order, Columbia, SC, USA
2 weeks ago •reported by user-zmxc7992
Unordered Package from Tech 1, 192-01 Northern Blvd, Flushing, NY 11358, USA
2 days ago •reported by user-xzmy6479
Received a keto pure bioribose that I did not order., Myrtle Point, Oregon, USA
6 days ago •reported by user-kczw1921