Recall notice
Walgreens Saline Nasal Spray WITH XYLITOL recalled due to Microbial contamination, United States
2 months ago •source accessdata.fda.gov
United States
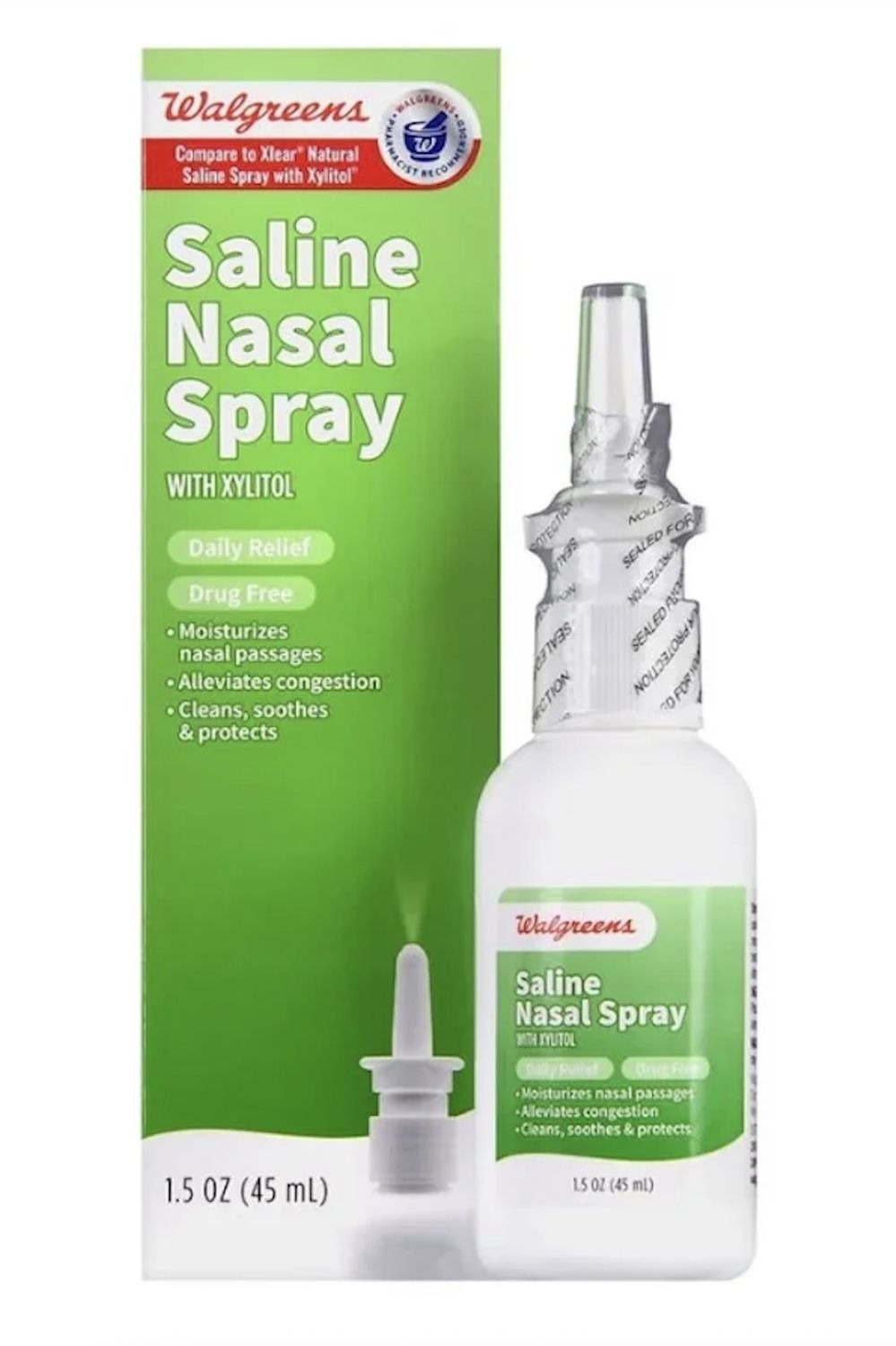
Medical Products Laboratories, Inc. is recalling Walgreens Saline Nasal Spray with Xylitol due to microbial contamination by Pseudomonas lactis. The product was distributed nationwide in the USA.AFFECTED PRODUCT:
- Walgreens Saline Nasal Spray WITH XYLITOL
- Each 1.5 oz (45mL)
- With lot numbers 71409 and 71861
- Expiring on 02/28/2027 and 08/31/2027, respectively.
- The product's NDC is 0363-3114-01.
- 41,328 bottles of the nasal spray
The issue was identified through internal testing, prompting a voluntary recall initiated on November 12, 2025. The recall is classified as Class II, with the classification date on November 21, 2025.
Source: www.accessdata.fda.gov/scripts/ires/
Comments
Comment