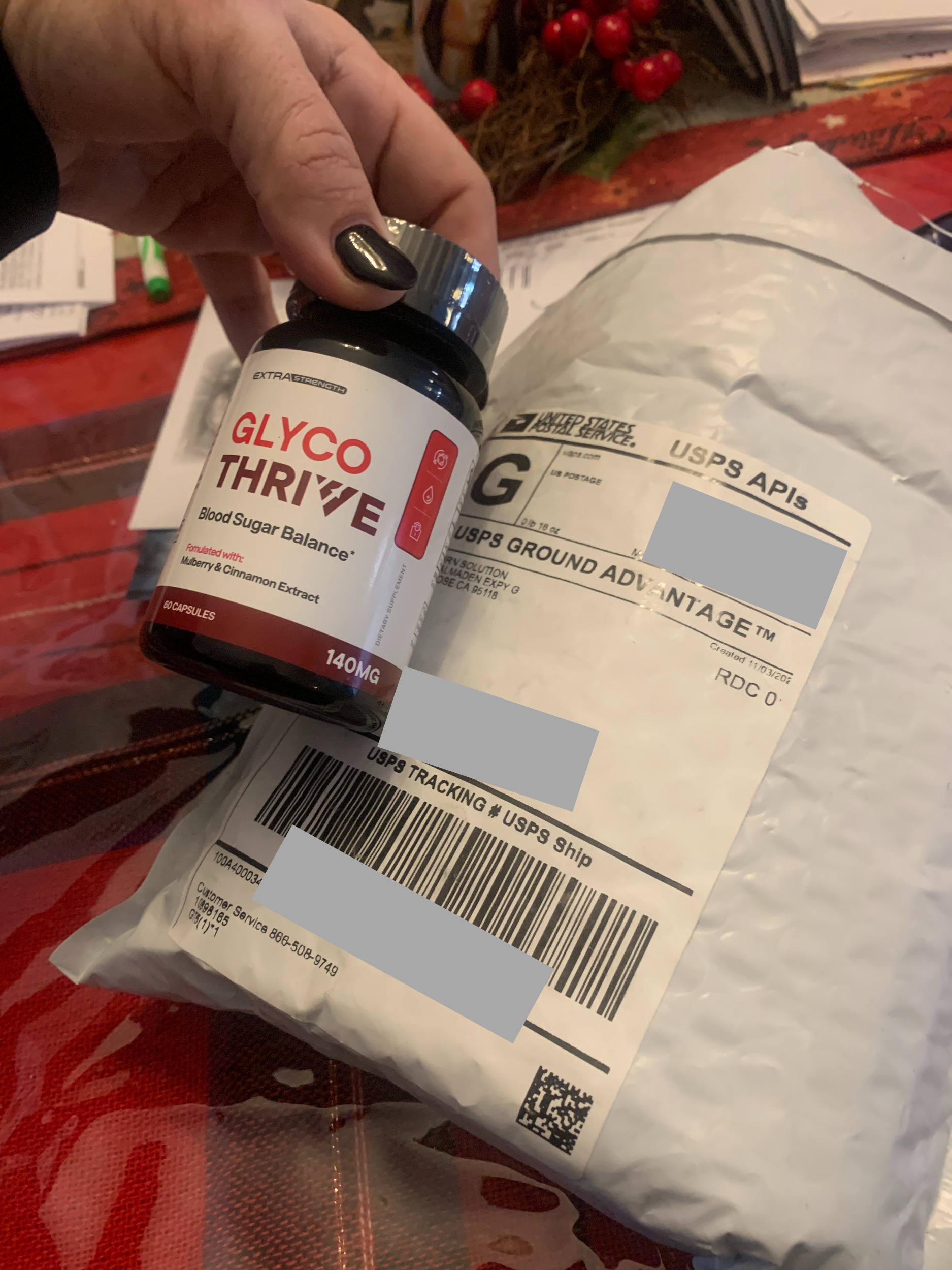
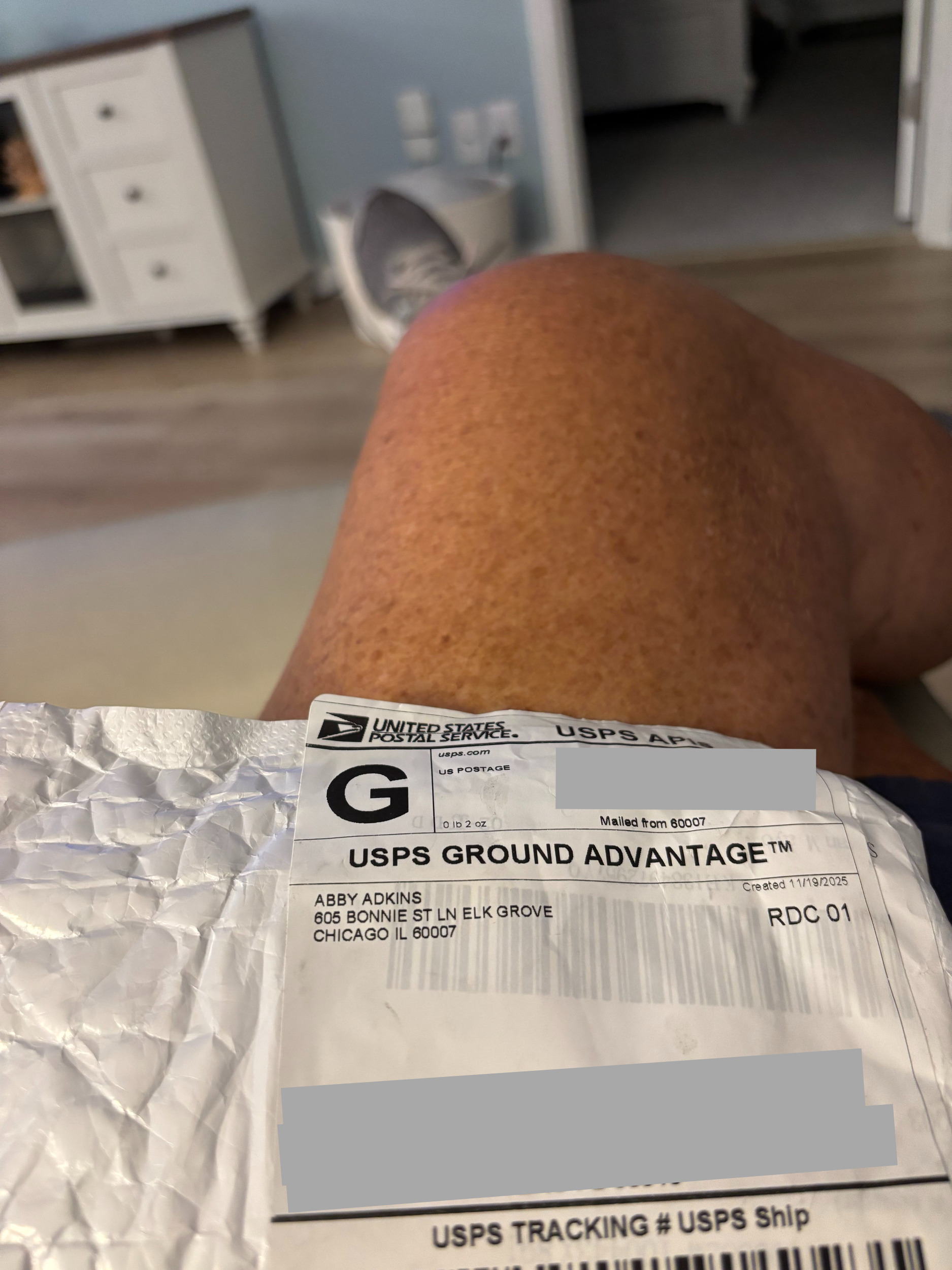
Scam! Ordered non invasive blood sugar monitors and got SaO2 monitors., Dallas, Texas, USA
2 months ago •reported by user-rgvm1583 • details
Company fraudulenty sent the wrong order. Problem is they won't return my money. Need to report them to the DA.