Brand name: Fresenius Kabi
Product recalled: Ketorolac Tromethamine Injection, USP, 30 mg/mL, and Ketorolac Tromethamine Injection, USP, 60 mg/2 mL
Reason of the recall: Presence of Particulate Matter
FDA Recall date: April 20, 2020
Recall details: Fresenius Kabi USA, LLC is
Particulate matter was found in eight reserve sample vials. Administration of products containing particulate matter could obstruct blood vessels and result in local irritation of blood vessels, swelling at the site of injection, a mass of tissue that could become inflamed and infected, blood clots traveling to the lung, scarring of the lung tissues, and allergic reactions that could lead to life-threatening consequences.
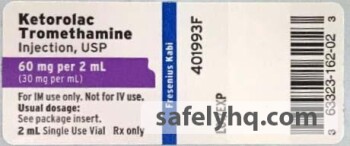
Ketorolac Tromethamine, a nonsteroidal anti-inflammatory drug, is indicated for the short-term (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level. The total combined duration of use of oral Ketorolac Tromethamine and Ketorolac Tromethamine injection should not exceed 5 days. Listed below is a table of the recalled lots distributed nationwide to wholesalers, distributors, hospitals, and pharmacies between May 5, 2018 and December 16, 2019, as well as a copy of the label:
- Ketorolac Tromethamine Injection, USP, 30 mg / mL, 1 mL fill in a 2 mL amber vial. NDC: 63323-162-01. Product code: 160201. (Check the recall notice for specific batch numbers)
- Ketorolac Tromethamine Injection, USP, 60 mg / 2 mL (30 mg / mL), 2 mL fill in a 2 mL amber vial. NDC: 63323-162-02. Product code: 160202. (Check the recall notice for specific batch numbers)
Check the full recalled product list and details on www.fda.gov
Source: FDA