Recall notice
Multiple Dietary Supplements recalled due to Undeclared Artificial Dyes, USA
8 months ago •source accessdata.fda.gov
United States
Preventics, Inc. is recalling multiple dietary supplements due to undeclared artificial dyes, Allura Red (Red No. 40) and Brilliant Blue (Blue No. 1), used in gelatin capsules. The products were distributed nationwide in the United States.The recall includes:
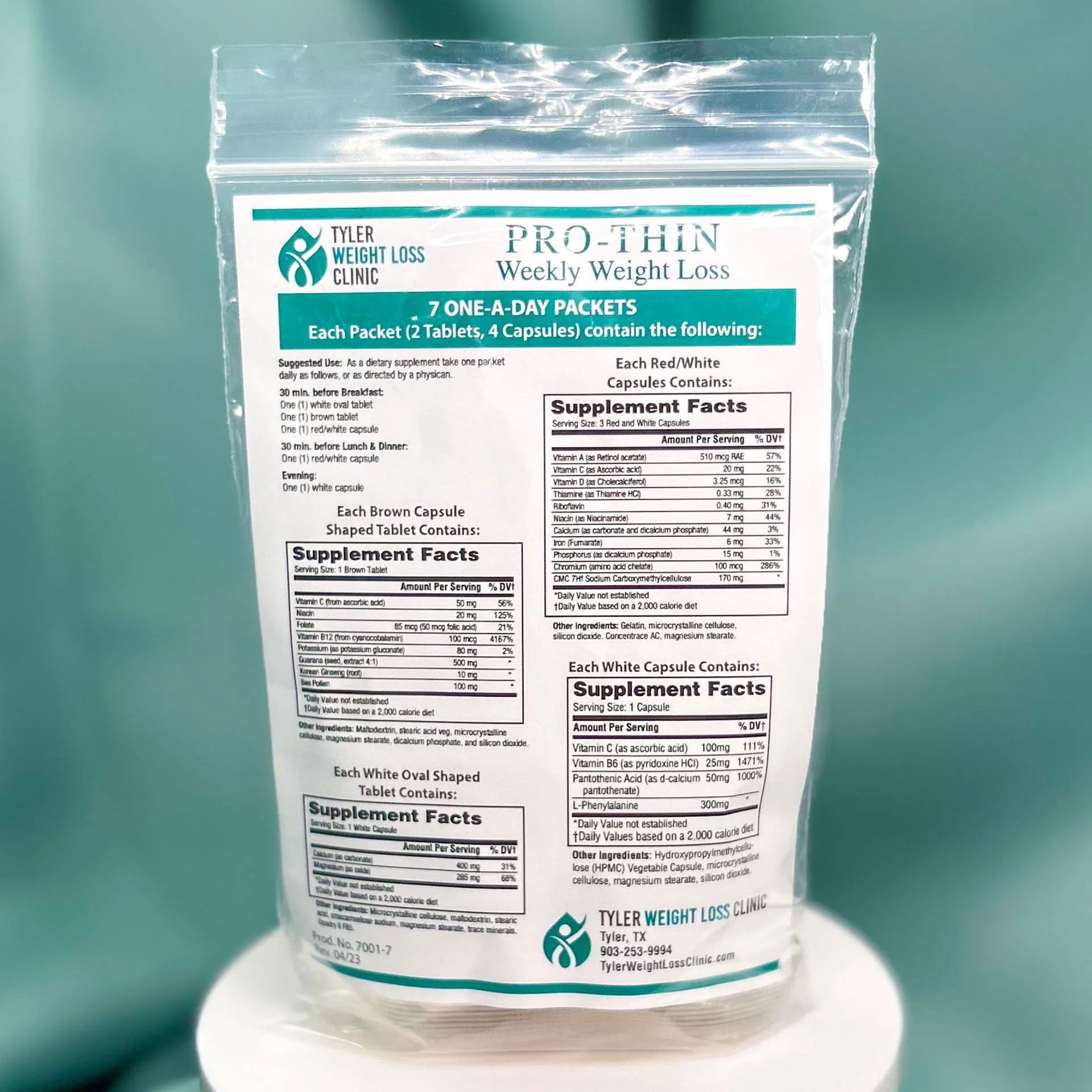
- Product: Prothin
- Description: Dietary supplement – Seven packets containing 2 tablets and 4 capsules each
- Product Quantity: 1,696 bags
- Code Information: 12131
- Product: Super ProTHIN Plus
- Description: Dietary supplement – Seven packets (2 tablets & 4 capsules each) plus one packet with 14 NutriLean capsules
- Product Quantity: 55,080 pills
- Code Information: 12131
- Product: Protrim Plus
- Description: Dietary supplement – 90 capsules per bottle
- Product Quantity: 763 containers
- Code Information: 21634-9
- Product: Probese Caps
- Description: Dietary supplement with Iron – 90-count bottles
- Product Quantity: 112,506 pills
- Code Information: 21874-19, 21874-20, 21874-21, 21874-22, 21874-23, 21874-24, 21874-25
- Product: NutriLean
- Description: Dietary supplement capsules
- Product Quantity: 94,190 capsules
- Code Information: 21745-11, 21745-12, 21745-13, 21745-14, 21745-15
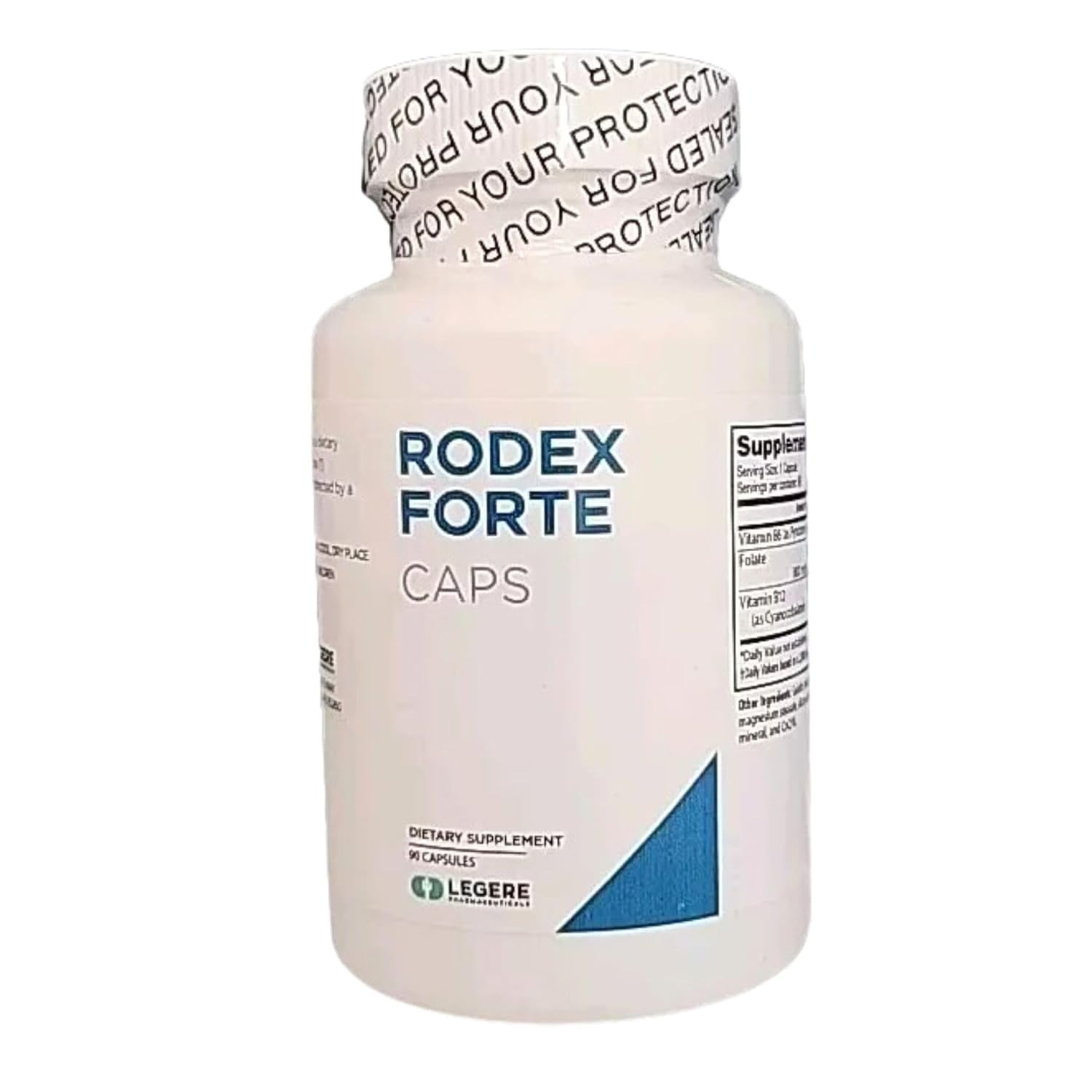
- Product: Rodex Forte
- Description: Dietary supplement – 90 capsules per bottle
- Product Quantity: 42,280 capsules
- Code Information (Batch codes): 21635-21, 21635-22, 21635-23, 21635-34
The recall was voluntarily initiated by Preventics on April 30, 2025, and classified as Class III on June 25, 2025. The issue was discovered through an internal review.
Source:
www.accessdata.fda.gov/scripts/ires/index.cfm
www.accessdata.fda.gov/scripts/ires/index.cfm
www.accessdata.fda.gov/scripts/ires/index.cfm
www.accessdata.fda.gov/scripts/ires/index.cfm
www.accessdata.fda.gov/scripts/ires/index.cfm
www.accessdata.fda.gov/scripts/ires/index.cfm
22
Comments
Comment