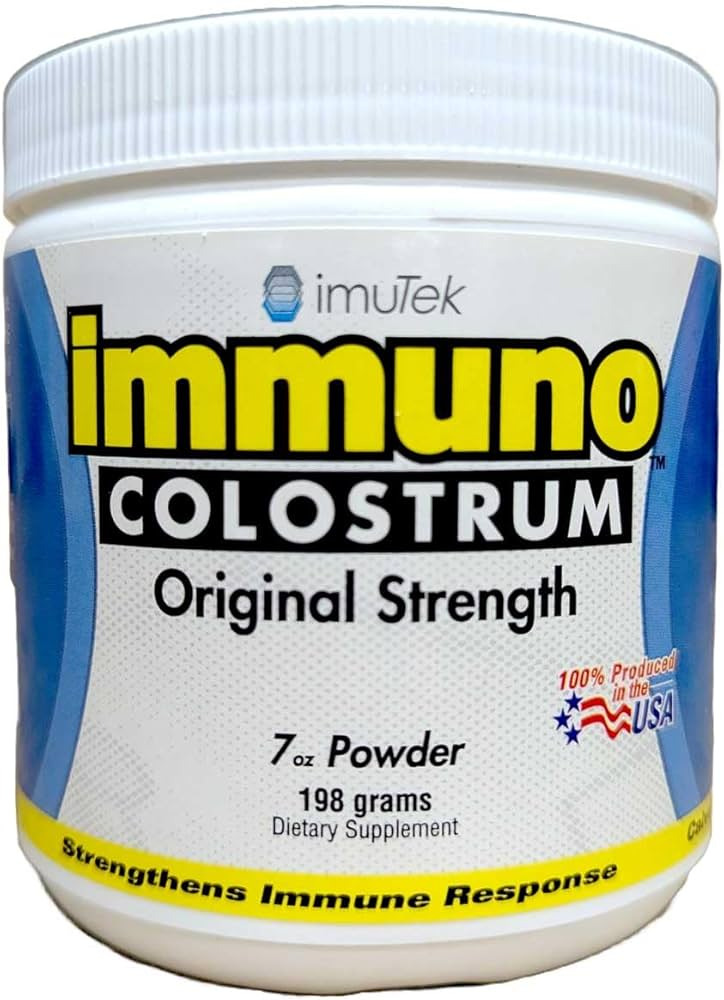
Recall notice
Imu-Tek Immuno-5 Colostrum Powder recalled due to Undeclared Allergen, United States
3 weeks ago •source accessdata.fda.gov
United States
Imu-Tek Animal Health, Incorporated has initiated a voluntary recall of its Immuno-5 Colostrum Powder due to an undeclared milk allergen. The product was distributed across several states, including AZ, CA, CO, FL, MA, ME, MN, NC, NH, OK, RI, TN, TX, UT, and WI in the United States.The recalled product is:
- The Imu-Tek Immuno-5 Colostrum Powder
- Packaged in a 7-ounce plastic jar with a sealed lid.
- It bears the UPC code 7 38654 00033 5
- Is labeled with lot number 216
- With an expiration date of 4/16/2027.
- A total of 113 bottles are affected by this recall.
The issue was discovered through internal testing, leading to the recall being classified as Class II. The recall was initiated on November 25, 2025, and classified on February 5, 2026.
Source: www.accessdata.fda.gov/scripts/ires/index.cfm
Comments
Comment