Recall notice
DermaRite Personal Care Products recalled due to Burkholderia cepacian contamination, USA
5 months ago •source fda.gov
United States
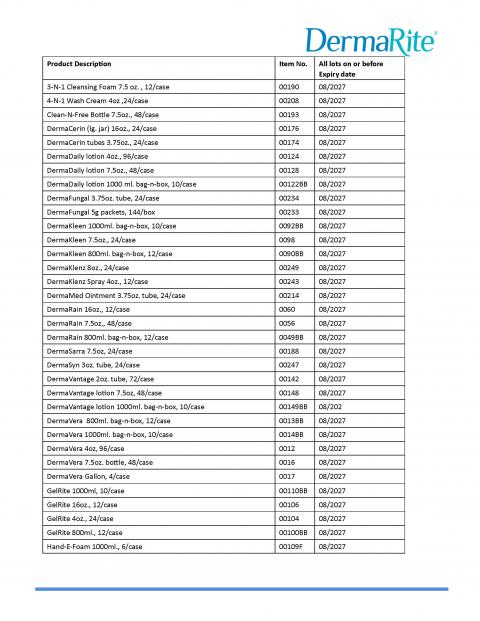
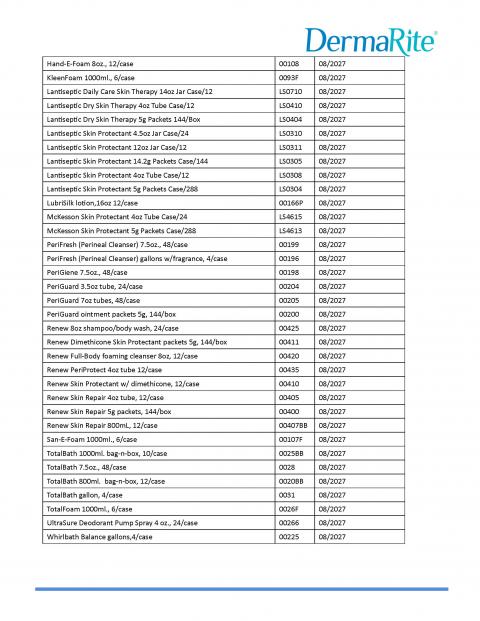
DermaRite Industries, LLC is expanding the voluntary recall initiated on July 16, due to potential microbial contamination identified as Burkholderia cepacia complex. Out of an abundance of caution the voluntary recall has expanded to include additional products and lots, which can be found in the table below. The recalled products were distributed nationwide in the United States and in Puerto Rico.The products included in this expanded voluntary recall include:
- 4-N-1 is a no rinse wash cream intended to temporarily protect and help relieve chafed or cracked skin
- DermaCerin is a skin protectant moisturizing cream indicated to temporarily protect and help relieve dry, chapped, or cracked skin. It also helps protect skin from the drying effects of wind and cold weather.
- DermaFungal is an OTC antifungal cream intended for the treatment and prevention of most athlete’s foot, jock itch, and ringworm. It relieves itching, scaling, cracking, and discomfort associated with these conditions.
- DermaKleen is an OTC healthcare antiseptic lotion soap with Vitamin E indicated for handwashing to decrease bacteria on the skin.
- DermaMed is an OTC skin protectant intended to dry the oozing and weeping of poison ivy, oak, or sumac, or other skin irritations.
- DermaSarra is an OTC external analgesic indicated for temporary relief of itching associated with minor skin irritations due to dry skin, insect bites, detergents, or sunburn.
- Gel Rite is an OTC instant gel hand sanitizer with vitamin E used to decrease bacteria on the skin. It is recommended for repeated use.
- Hand E Foam is an OTC foaming hand sanitizer with vitamin E used for handwashing to decrease bacteria on the skin. It is recommended for repeated use.
- KleenFoam is an OTC antimicrobial foam soap with Aloe Vera indicated for handwashing to decrease bacteria on the skin after changing diapers, after assisting ill people, or before contact with a person under medical care or treatment.
- Lantiseptic is an OTC skin protectant indicated to temporarily protect minor cuts, scrapes and burns. It helps prevent and temporarily protect chapped or cracked skin.
- PeriGiene is an OTC antiseptic cleanser indicated for use in the perineal area.
- PeriGuard is an OTC skin protectant indicated to help relieve and prevent rashes and irritation due to wetness from incontinence. It also protects chafed skin due to irritation and helps seal out wetness.
- Renew Dimethicone is an OTC skin protectant indicated to help treat and prevent diaper rash, protect minor skin irritations associated with diaper rash, and helps seal out wetness.
- Renew Periprotect is an OTC skin protectant indicated to help treat and prevent diaper rash, protect minor skin irritation associated with diaper rash, and helps seal out wetness.
- Renew Skin Repair is an OTC Skin cream indicated to temporarily protect and help relieve chapped or cracked skin. It is beneficial for face, hands, body and legs.
- UltraSure is an OTC anti-perspirant & deodorant indicated to reduce underarm wetness.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/dermarite-industries-expands-voluntary-nationwide-recall-due-potential-burkholderia-cepacia
Comments
Comment