Recall notice
Bottles of Bariatric Fusion Iron Multivitamins recalled due to Risk of Serious Injury or Death, United States
4 months ago •source cpsc.gov
United States
Blueroot Health has issued a recall for its Bariatric Fusion Iron Multivitamin bottles due to non-compliance with child-resistant packaging standards, posing a risk of serious injury or death from child poisoning. These products were sold online in the United States, including on Amazon and Bariatricfusion, from January 2025 to June 2025. Approximately 4,700 units are involved in this recall.The recall affects:
PRODUCT: Bariatric Fusion High ADEK Multivitamin Capsules
- Size/Capsules: 90-count and 270-count bottles
- Packaging: Bottle
- Color: White and orange
- Lot Number: 0066J4, 0065J4, 0453B5, 0370B5
- Additional Information: Only bottles with smooth cap tops that do not have the “push down & turn” embossed lettering are affected. The Bariatric Fusion logo is printed on the front of the bottles.
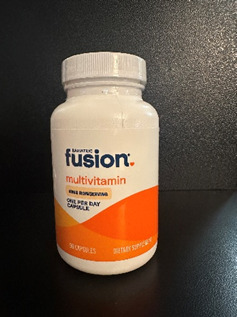
PRODUCT: Bariatric Fusion One Per Day Bariatric Multivitamin Capsules
- Size/Capsules: 90-count bottle
- Packaging: Bottle
- Color: White and orange
- Lot Number: 0066J4, 0065J4, 0453B5, 0370B5
- Additional Information: Only bottles with smooth cap tops that do not have the “push down & turn” embossed lettering are affected. The Bariatric Fusion logo is printed on the front of the bottles.
The issue was identified due to the absence of child-resistant caps, violating the Poison Prevention Packaging Act. The recall was initiated on September 11, 2025.
Source: www.cpsc.gov/Recalls/2025/Blueroot-Health-Recalls-Bottles-of-Bariatric-Fusion-Iron-Multivitamins-Due-to-Risk-of-Serious-Injury-or-Death-from-Child-Poisoning-Violates-Mandatory-Standard-for-Child-Resistant-Packaging-Manufactured-by-VitaQuest-International
21
Comments
Comment