Source: Osha.gov | Receipt Date: 2021-09-20
Harris teeter
Updated:
Source: Osha.gov | Receipt Date: 2021-09-20
Covid-19 OSHA Complaint, Harris Teeter, LLC - Store 280, 4010 Battleground Ave., GREENSBORO, NC, 27410, USA
3 years ago
Source: Osha.gov | Receipt Date: 2021-02-02
Covid-19 OSHA Complaint, Harris Teeter, LLC, 3040 Evans Street, GREENVILLE, NC, 27858, USA
3 years ago
Source: Osha.gov | Receipt Date: 2020-12-22
Covid-19 OSHA Complaint, Harrah's Lake Tahoe, 15 U.S. 50, Stateline, NV 89449, United States
3 years ago
2. The employer has not implemented or conducted daily surveys for changes to employee?s health conditions to prevent the spread of Covid-19. 3. The employer …
Alleged Hazards: 3,
Source: Osha.gov | Receipt Date: 2020-06-08
Covid-19 OSHA Complaint, Harris Teeter Pharmacy, 10060 Market Circle, Manassas, Virginia 20110, USA
3 years ago
Alleged Hazards: 1, Employees Exposed: 80
Source: Osha.gov | Receipt Date: 2020-09-17
Covid-19 OSHA Complaint, Harris Teeter, 2800 S College Rd, Wilmington, North Carolina 28412, USA
3 years ago
2. Employees are not aware of the employer's written hazardous communication program nor are they provided safety data sheets "SDSs" for chemicals they are exposed to in the workplace to include the unknown chemical sprayed by the company for COVID 19.
3. The back receiving area is very hot, around 97 degrees; and employees have not been provided information on signs and symptoms of heat stress; and means of preventing heat stress and other heat related illness through their heat stress prevention program.
Source: Osha.gov | Receipt Date: 2020-07-07 | Symptoms: Skin Rash, Sore Throat
Recent Interesting Reports
Silver skull with flower ring, Oxnard, CA, USA
3 weeks ago •reported by user-cvmy2621
I purchased 5 of your large lighter smoking sets and none of them light., York, PA, USA
4 weeks ago •reported by user-wdvb9628
Brushing scam from Jane, 371 Little Falls Road, Cedar Grove, NJ, USA
3 weeks ago •reported by user-vpnxd669
AuroMedics Pharma Methocarbamol Injection - recalled due to Particulate Matter presence, USA
3 weeks ago •source www.fda.gov
Recall notice
Administration of an injectable product that contains particulate matter may result in local irritation or swelling. If the particulate matter reaches the blood vessels or is injected intravascularly it can travel to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. To date, Eugia US LLC has not received any reports of adverse events related to this recall.
Methocarbamol injection USP 1000 mg/10 mL (100mg/mL), is used as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. It is packaged in 10 mL and packed as 25 (vials) X 04 (Printed E-Flute cartons) X 01 (Shipper) with NDC code as 55150-223-10. Eugia US LLC shipped the entire lot to wholesalers nationwide from Jan 12, 2024, through Jan 16, 2024.
The product can be identified by product name on carton and vial label and with lot number 3MC23011 and Exp. Date: Nov 2026 (NDC 55150-223-10) (See enclosed vial label). The product label is as shown in the image below.
Eugia US LLC (f/k/a AuroMedics Pharma LLC) is notifying its distributors by recall letters and is arranging for the return/replacement of all recalled products. Wholesalers, hospitals, pharmacies, institutions, and doctors with an existing inventory of the recalled product lot should discontinue use, stop distribution and quarantine the product immediately. If you have further distributed the recalled product lot, notify your accounts and/or any additional locations which may have received the recalled product. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
If you are experiencing any harm after using this product it is important to report it. It can help to detect & resolve outbreaks early and prevent others from being harmed, and it enables better surveillance. If symptoms persist seek medical attention.
Company name: Eugia US LLC
Brand name: Eugia US LLC
Product recalled: Methocarbamol Injection, USP 1000 mg/10 mL (100mg/mL) (Single Dose Vial)
Reason of the recall: Device & Drug Safety – Presence of Particulate Matter
FDA Recall date: March 28, 2024
Source: www.fda.gov
I did not order this product, Greenbelt, Maryland, USA
1 week ago •reported by user-hnby3436
I received a parcel for 2 bars of soap that I did not order., Rancho Cucamonga, CA, USA
4 weeks ago •reported by user-jxwc6441
I keep getting Keto acv Gummies that I didn't order, Homegardner Road, Castalia, OH, USA
1 week ago •reported by user-xthmq334
192-01 Northern Blvd
Flushing Ny. 11358 "STOP "
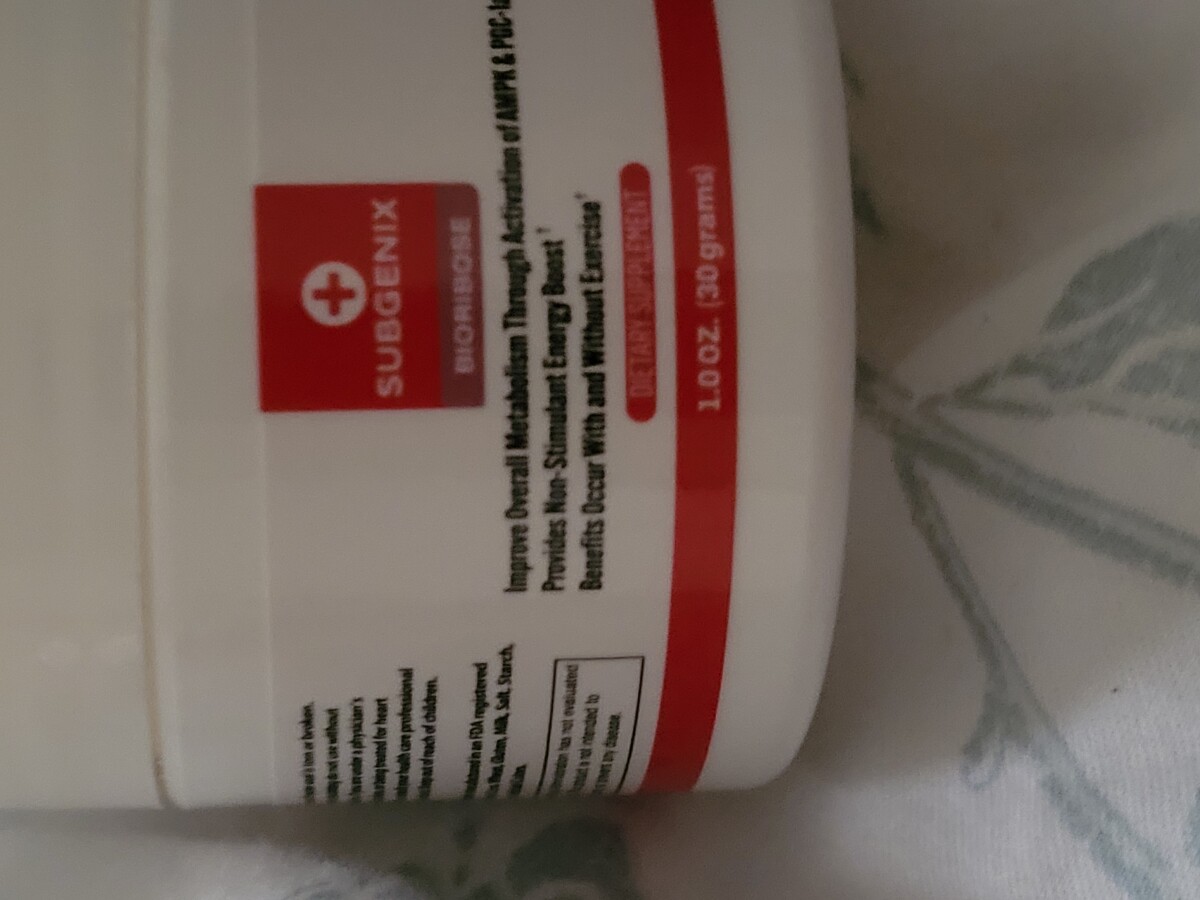
I just received this package also from Tampa Florida... Subgenix, Houston, TX, USA
2 weeks ago •reported by user-fkkg2382
Empty package, Fishing Creek, MD, USA
1 week ago •reported by user-gnxv8262
Unordered package, Ontario, Canada
3 weeks ago •reported by user-yjcbc883
received by Logistic 895 Meyerside Dr MISSISSIGA ONTARIO CANADA. JY243510U16208****
Report by
IMPORTANT - YOUR REPORT IS QUEUED - IT MAY TAKE UP TO 12 HOURS FOR YOUR REPORT TO SHOW ON OUR HOME PAGE (IF NOT OPTED AS PRIVATE)
Visit our learn pages for more helpful information or, email us: support@safelyhq.com